Thermal energy is an inner energy type. The thermal energy is operated and manifests as the spontaneous movement of the atoms and small particles by all subject matter. The level of thermal energy depends on the subject’s temperature.
A simplified definition for temperature:
Temperature is a measure of the degree of hotness or coldness of a body.
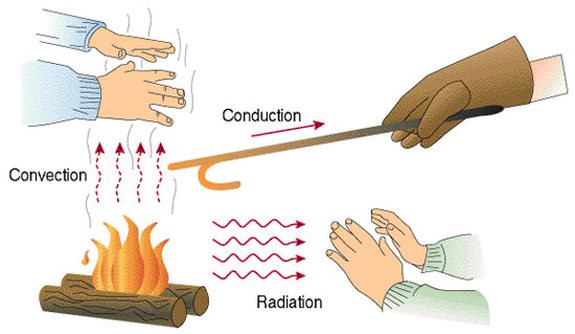
- Due to variations in temperature through thermal conductivity, convection, and radiation, thermal energy may be transferred from one area to another. Thermal control transfers from a higher temperature object to a lower temperature object (will explain these three processes later). However, when all things enter thermal equilibrium at the same temperature, this exchanging of heat ceases.
- The overall energy in a body isn’t the same as the temperature. Other sources of energy often provide the total energy found in the body.
- In thermal interaction, two objects are assumed to be thermally balanced with one another if the heat transfer between them is not net.
- The hotter body becomes colder in thermal touch and the colder body becomes warmer until there is no more transition.
- The two objects are considered to be in thermal balance at the same location.
(The Planet is in thermal contact for example, while the Planet clearly does not impact the Sun’s atmosphere, so actual structures (not idealized) are still in thermal contact. (The objects are supposed to be in thermal contact, but the Ears are not in thermal contact with the Sun.)
Thermal touch is not thermal harmony. The Planet has thermal interaction with the Sun but is not in thermal balance.
Read also: Temperature
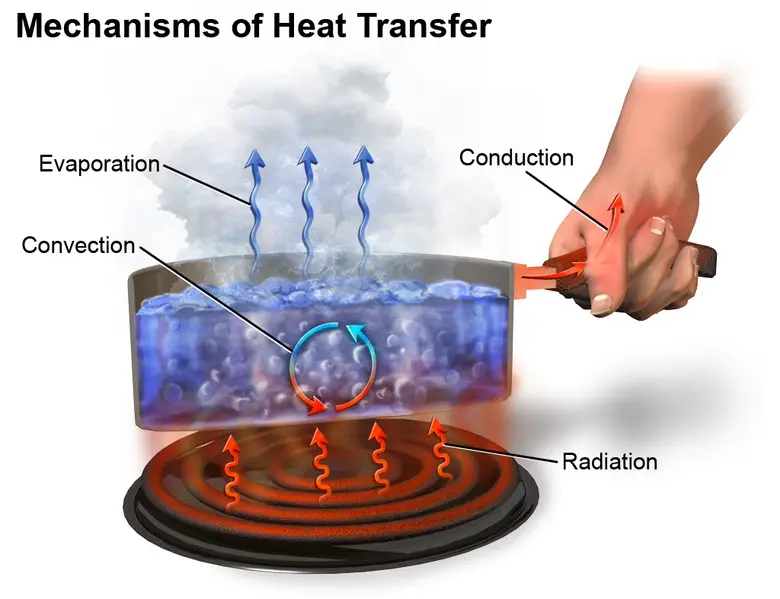
Conduction:
Conduction is the diffusion of thermal energy without material flux from one location to another.
Healthy thermal conductors are metals. Efficient thermal conductors are non-metals (plastics). (Thermal insulators) (They are)
In the process of actions, artifacts usually need physical interaction.
Mechanisms of conduction:
1. Atomic Collisions
- Vibrate regarding its fixed location in solids, atoms, or molecules. In cooler region atoms or molecules are vibrating more or more intensely than in the colder region or having more kinetic energy.
- Ses molecules collide with their neighbors and give them some cinematic electricity. The neighbors conflict with their neighbors. Thus, heat is brought to cooler areas and the temperature is increased.
- This method is long.
- Owing to the tightly wrapped molecules, solids conduct heat better than liquids and gases.
2. Diffusion of Free Electron
- In comparison to nuclear collisions, the large quantities of free electrons usable for thermal conduction are known as thermal drivers. Free electron diffusion is defined as the migration of fast-moving electrons.
- The lack of free electrons in rigid thermal insulators limits the thermal conduction of atoms and molecules in the crystal lattices to vibrations.
- It’s an incredibly fast operation.
Applications of conduction:
Cooking pans are often made with metals because of their good thermal conduction property. In contrast, the handles of the cooking utensils are made up of insulators to protect the hands from scalding.
Sawdust is used to cover the ice cubes from melting.
Convection:
Convection is the transfer of thermal energy from one place to another using bulk fluid movement in the material medium.
In fluids, thermal energy transfer is mainly through thermal convection. In solids, since its substance cannot flow, there can be no thermal convection.
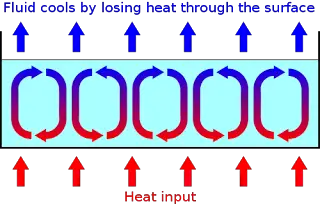
Mechanism:
In convection, the movement occurs as a result of gravity. The hot part of the fluid expands becomes less dense and rises. It is displaced by the colder, denser part of the fluid, which in turn is heated up. This fluid movement or convection current allows heat to be transported.
Applications of Convection:
In a refrigerator, convection is used to circulate cold air around the food. Air is cooled by the freezer compartment at the top of the refrigerator. As it sinks, it is replaced by warmer air rising from below. The circulating air carries away heat energy from all the food in the fridge.
Read also: Static Electricity
Radiation:
Radiation is the transmitting of thermal energy through electromagnetic radiation from one location to another, without the need for a material media that intervenes.
All material radiates thermal energy in all directions at temperature-specified quantities, where the energy is provided by the electromagnetic spectrum’s infrared, visible and x-ray components. These photons heat everything that takes them further.
The only mechanism not needed to pass power is radiation.
Factors affecting the rate of radiation:
Color and texture of the surface
Black, matt surfaces are good in both absorbing and emitting radiation.
Shiny or polished white surfaces are poor absorbers because they act better as reflectors, and hence, poor emitters.
Surface temperature
The hotter the object, the more energy it radiates
Surface area
The greater the area, the more energy it radiates.
Applications of radiation:
The greenhouse effect offers a way of growing plants in cold countries that require a warm climate. Quick infrared sunlight travels through the greenhouse’s glass windows, is absorbed by inside plants and soil. The plants still radiate light, but they have a much longer wavelength. The glass panels reflect this radiation. Thus, the temperature inside the greenhouse rises to a thermal balance ideal for the growth of plants.
Under the roof tiles is a coating of aluminum sheet to preserve the ambient temperature inside the house. The aluminum sheet mirrors the radiation day by day and makes the framework colder. In the night, radiation is reduced from inside and the interior is hot.
Vacuum Flask:
A vacuum flask can store and maintain the temperature (either hot or cold) of the contents in the flask by reducing heat transfer in or out through conduction, convection, and radiation.
| Type of heat transfer | How heat transfer is reduced |
| Convection | Vacuum between the double glass walls |
| Conduction | Vacuum between the double glass walls.
Insulated cover and stopper |
| Radiation | The shiny silvered inner surface of the glass walls |

