Atom isotopes (radioisotopes) might be stable or unstable. An unstable nucleus IF it has too many neutrons and/or protons. By ejecting electrons, the unstable nuclei can attempt to become stable. Radioactivity is termed the method of ejecting the particles.
Radioactive particulate emission from the mass nucleus is produced by alpha particles or beta particles, and is often accompanied by energy emission by gamma rays.
Radioactive decay is a random and accidental process, through emission of alpha particles (helium nuclei), beta particles (electrons) and/or gamma radiation (cort-wavelong electromagnetic waves), whereby an unstable center disintegrates into a more stable state.
Radioactive emissions can be detected using the following common detectors:
Photographic detectors
When a radioactive substance is placed near a photographic film coated with silver halide, the latter will produce a similar effect as seen with exposure to visible light.
After the exposure is completed, this latent record of the accumulated exposure can be made visible through a chemical development process.
Geiger-Muller Tube (GM-tube)
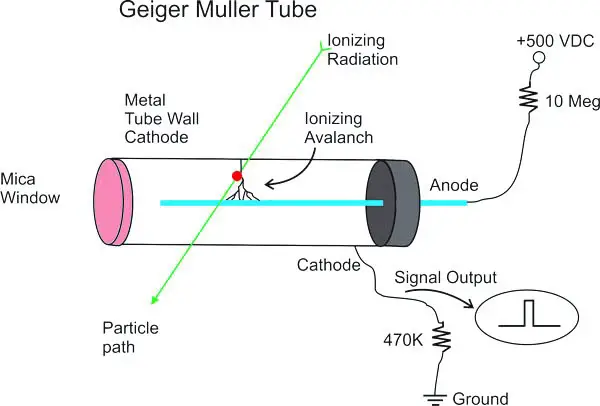
The most useful radiation detector used in the calculation or estimation of the power of a radiation pulse is a GM tubing. As radiation from the thin window of the mica reaches the tube, it hits and ionizes big Argon atoms.
The free electrons then speed up in the direction of the fine wire anode positioned along the cylindrical cathode axis. These accelerated electrons allow the argon atoms to ionize more by colliding with them to create a ‘avalanche’ of the electrons collected nearly instantly by the anode.
The ions are positively charged to the cathode. A new pulse comes out throughout the electrode array of the electrons and argon ions. The current pulse is then extended and supplied to a rate meter.
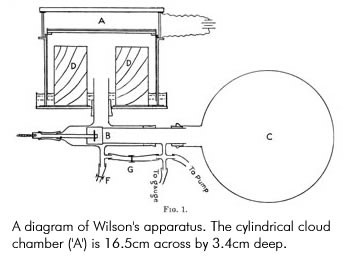
Diffusion Cloud Chamber
When emissions from the radioactive substance are allowed to come into contact with air molecules, it will tend to knock out the electrons of the molecules along its paths, causing ionization (i.e., the air molecules are electrically imbalanced and become ions).
The diffusion cloud chamber is the device for making visible the paths of ionizing emissions.
It consists of a container containing air. At the top of the chamber, a row of felt strips is soaked in ethanol (alcohol). The lower part of the chamber is cooled by solid carbon dioxide or liquid helium. The alcohol evaporates into vapor and continuously diffuses downwards. It then becomes supersaturated at the center of the chamber (i.e., the air holds more vapor than it normally could).
When emissions from the radioactive substances are placed in the chamber, the emissions will cause ionization along its paths. The ionized air molecules will attract the vapor molecules and become too heavy to stay in suspension and thus, condense into tiny liquid droplets along the paths of the emissions.
The randomness of radioactive emissions
No chemical reactions or physical changes such as temperature, strain, electrical or magnetic fields and so forth shall influence the radioactivity.
Both radioactive element core nuclei are with the same possibility with nuclear degradation (emittance of alpha, beta or gamma). The next nuclei to decay are not a question, or how long it takes for decay. Unlikely.
Since the decay is entirely spontaneous regardless of ambient conditions and the nucleus, i.e. radiation releases arise in a random way over time and space.
It is noted, however, that the average of its radioactive emissions does conform with some statistical rules for several regular measurements of the random method. About how many radioactive compounds are going to degrade in X time. That can be measured.
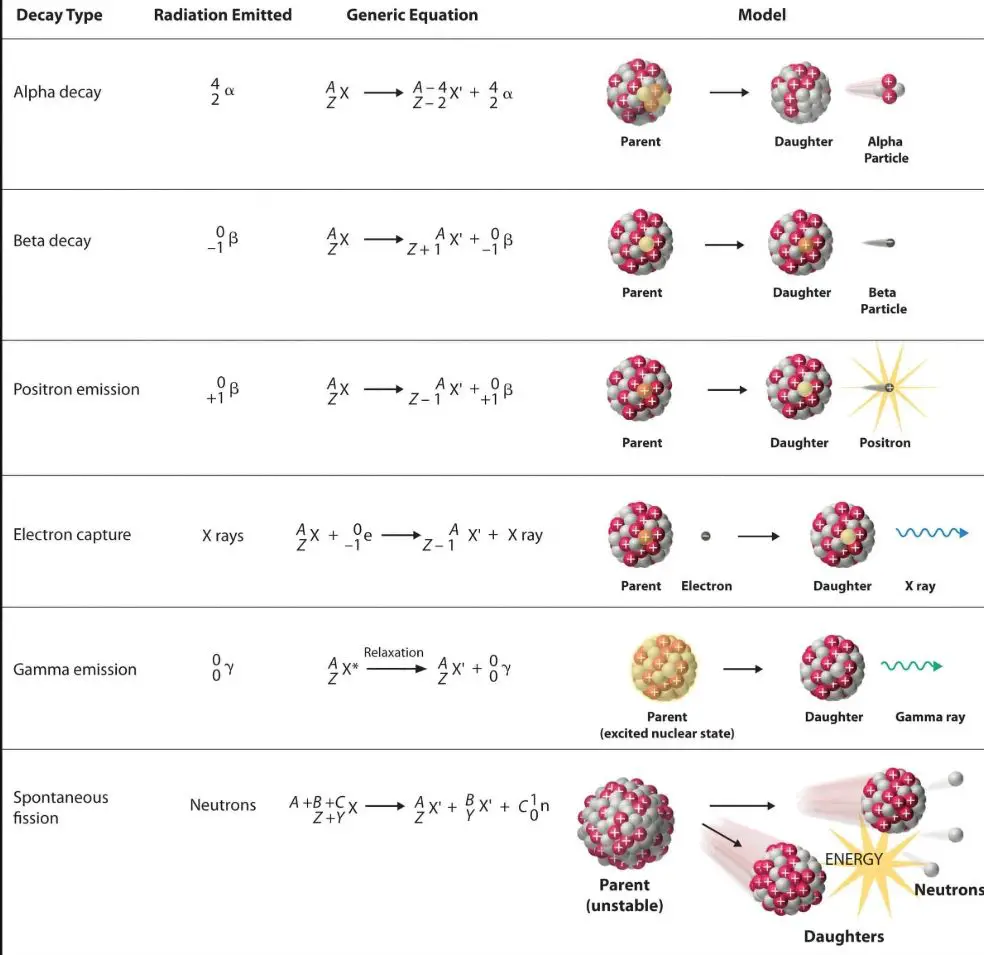
Characteristics of three types of emission
The α-particles are made up of 2 protons and 2 neutrons, which is similar to the helium nucleus. The weight is 4u (atomic mass units 4 / proton mass) and +2 e load. α-particles fly at about 1/10 of the light speed.
β-particles are electrons that are released at various speeds and can be as high as 9-10ths of light speed. They bear negative loads of -e like electrons. β-particles are significantly stronger than α-particles. (11836 the proton’s mass).
μ-rays are very low range of electric radiation. Sometimes they are released concurrently with α- or β- particles. Small rays are massless. Massless.
Ionization
Ion is a charged atom, formed by losing or gaining electrons. All three kinds of emissions are capable of creating ions.
α-particles knock electrons out of nearby atoms, thus ionizing the atoms. The α-particles are much heavier than the electrons and can simply dislodge them from the atoms, thus they have the most powerful ionization ability.
β-particles are less strongly ionizing than α-particles, but more than γ-rays. It is less likely for a lighter β-particles to dislodge an electron.
γ-rays are the least ionizing of the nuclear radiation as it is uncharged.
Penetrating power
α-particles are the least penetrating of the nuclear radiations and can be stopped by a sheet of paper. They can only pass through a few cm of air.
β-particles are more penetrating and are stopped by a thin (1 mm) sheet of aluminum. The fastest β-particles have a range of a meter or so in air.
γ-rays are the most penetrating nuclear radiation. Intensity of gamma radiation can be reduced to half by 1 or 2 cm thick lead.

