What is the Kinetic Model of Matter?
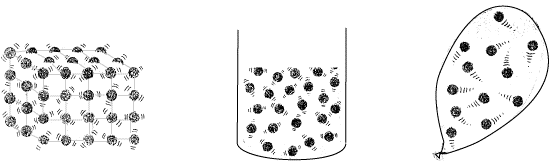
The kinetic theory is that all matter is made up of a vast number of small particles (atoms or molecules), all of which are in continuous, spontaneous movement. The idea is kinetic or kinetic molecular.
The fast traveling particles collide continuously with each other and the container walls. The kinetic theory describes when observing the structure and motion of the molecular gases, macroscopic properties, such as friction, temperature, or volume.
In essence, the principle says that strain is not due to static repulsion, as Isaac Newton had conjectured, between molecules traveling at different velocities. In short, philosophy leads to understanding the behavior. The movement or transfer of heat and the link of pressure, temperature, and volume properties of the gases are two important areas explained.
Solid
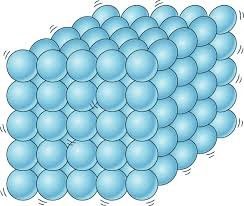
The strong attractions between the particles hold closely together in a solid. Although they vibrate, it is not necessary to disturb the structure.
A lot of thermal energy is then required to pull the particles apart.
This explains why Solids have High Melting Point.
Process of Melting of solid:
The particles gain energy as a solid is heated and start vibrating faster and faster. The foundation is initially weakened slowly, which allows the solid to extend. Additional heat produces more energy before the particles tend to break loose. And if the particles are already loose, they will move around. The solid melts into a liquid at this point. The ions in the liquid have more energy than the solid. To melt a solid, energy is important to resolve the attractions of the particles and to make it possible to tear them apart.
Solids have a fixed surface and volume (at a particular temperature) because of the strong particle attraction.
Solids will expand a little on heating but nothing like as much as liquids because of the greater particle attraction restricting the expansion and contraction occurs on cooling.
The expansion is caused by the increased energy of particle vibration, forcing them further apart causing an increase in volume and a corresponding decrease in density.
Read also: Forces
Arrangement of Particles
Solids cannot be compressed because the molecules are arranged close together in a regular pattern and there is little space between them. With an increase in temperature, the molecules gain kinetic energy and vibrate more. The separation between molecules increases slightly and the solid expands. Solids have the greatest density (‘heaviest’) because the particles are closest together. Solids are extremely difficult to compress because there is no real ‘empty’ space between the particles.
Motion
Specific molecules are trapped in close positions and cannot move outside of each other. The strong atoms or molecules are still in motion.
- Sections of the solids therefore continuously vibrate due to their inner force in their set positions but cannot move from place to place. Sections of solids contain only the potential of vibration.
- Solids are not able to flow as freely as gases or liquids because the particles are held in fixed positions.
- The diffusion of solids is almost impossible because the ions are very densely packed and kept together without “dead space.”
Brownian motion
Extreme, intermittent acceleration of gas and fluid molécules is Brownian motion. Brown’s motion simply demonstrates the dynamic molecular model of matter, where small particles, with a wide variety of speeds n all directions, and kinetic energies, are in constant random movement.
In the aforementioned simulation, the particles are seen spontaneously traveling.
Why do the particles move randomly?
Air molecules are bombarded due to the unusual passage of particles detected. So fragile are the air molecules to look at. In both directions, air molecules constantly attack the particles unevenly. This allows the smoke particles to move irregularly.
The spontaneous movement of particles shows that air molecules travel spontaneously with a variety of speeds and kinetic energies in all directions.
The brownish activity of the particles is more frenzied as the temperature increases. They are going to be more energetic.
Liquids
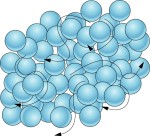
Particles of liquids are kept together by forces of attraction that are weaker than those of solid particles.
Often in liquids, intermolecular forces (such as the hydrogen bonds are shown in the animation below) pull molecules together and are quickly broken.
Liquids will expand on heating but nothing like as much as gases because of the greater particle attraction restricting the expansion (will contract on cooling).
When heated, the liquid particles gain kinetic energy and hit the sides of the container more frequently, and more significantly, they hit with a greater force, so in a sealed container the pressure produced can be considerable!
Liquid also cannot be compressed as the molecules are still close together and there is little space between them. When a liquid is heated, the molecules vibrate and move about more vigorously. Thus the liquid expands, but only very slightly. Hence, more thermal energy is required to pull particles of liquid apart.
This results in Liquids having a lower Melting point and boiling point than that of a solid
Liquids have a surface, and a fixed volume (at a particular temperature) because of the increased particle attraction, but the shape is not fixed and is merely that of the container itself.
Liquids seem to have a very weak ‘skin’ surface effect which is caused by the bulk molecules attracting the surface molecules disproportionately.
Arrangement of particles
The molecules are not arranged in a regular pattern; randomly arranged and are slightly farther apart than in solids. Though there are still forces between the molecules, they are not held in fixed positions.
Hence,
Liquids have a much greater density than gases (‘heavier’) because the particles are much closer together because of the attractive forces.
Liquids are not readily compressed because of the lack of ‘empty’ space between the particles and as the molecules are still close together and there is little space between them. When a liquid is heated, the molecules vibrate and move about more vigorously. Thus the liquid expands, but only very slightly.
Motion
In liquids, molecules can move past one another and bump into other molecules; however, they remain relatively close to each other like solids. Liquids usually flow freely despite the forces of attraction between the particles but liquids are not as ‘fluid’ as gases. Liquids have a surface, and a fixed volume (at a particular temperature) because of the increased particle attraction and, but the shape is not fixed and is merely that of the container itself.
(Within the walls of the container they can move from place to place bumping into the sides of the container and other particles. This type of energy is called translational energy. This energy gives a liquid the ability to flow and be poured and to spread when a liquid is spilled. Liquid particles also have vibrational energy.)
Liquids seem to have a very weak ‘skin’ surface effect which is caused by the bulk molecules attracting the surface molecules disproportionately. The natural rapid and random movement of the particles means that liquids ‘spread’ or diffuse. Diffusion is much slower in liquids compared to gases because there is less space for the particles to move in and more ‘blocking’ collisions happen.
Read also: Energy, Work, Power
Gases
Particles of gases are “more rarefied” than either liquids or solids. This means that the forces of attraction that hold them together are very Gases have no surface, and no fixed shape or volume, and because of lack of particle attraction, they always spread out and fill any container (so gas volume = container volume). A gas has no fixed shape or volume but always spreads out to fill any container.
Arrangement Of Particles
The particles are widely spaced and scattered at random throughout the container so there is no order in the system.
The Particles occupy any available spaces
Gases have a very low density (‘light’) because the particles are so spaced out in the container (density = mass/volume).
Density order: solid > liquid >>> gases
Gases are readily compressed because of the ‘empty’ space between the particles.
Ease of compression order: gases >>> liquids > solids (almost impossible to compress a solid)
Motion
The particles move rapidly in all directions, frequently colliding with each other and the side of the container.
With an increase in temperature, the particles move faster as they gain kinetic energy.
Gases flow freely because there are no effective forces of attraction between the gaseous particles – molecules.
Ease of flow order: gases > liquids >>> solids (no real flow in solid unless you powder it!)
Because of this gases and liquids are described as fluids.
Particles Move at high speeds randomly so they have no fixed shape or volume, but always spreads out to fill any container.
EVIDENCE IN SUPPORT OF RANDOM MOTION OF GAS MOLECULES
The striking evidence of the molecular agitation of matter comes from such physical phenomena as diffusion, evaporation, Brownian motion, etc.
(a) Brownian Motion
It’s called Robert Brown, the English botanist. In 1827, he pointed out the wave. He noticed that pollen in water if examined under a powerful microscope, displays a persistent unorderly flow. Brownian motion is the erratic acceleration of suspended objects. Only after the advancement of the cinematic theory was the right interpretation conceivable for the Brownian motion.
It seems crazy that millions of molecules pass about in it as we look at a bottle of water already on the surface. However, more than 200 years ago Robert Brown finds proof of the revolution. He detected very small particles of pollen trapped in water through a microscope. To his delight, he observed a constant haphazard movement of some of the smaller particles. This is how the liquid ions everywhere over them bombard small particles. The resulting force is still shifting in the course.
Experiment:
Brownian motion can be demonstrated simply by releasing some smoke particles from the burning cord into a small glass container and putting a cover plate to seal the smoke and air into the cell.
To investigate liquid molecular movement, place some water with graphite particles suspended in it in the cell.
Now adjust the microscope slightly until you can see very bright specks. The particles of graphite (or smoke) scatter (reflect) the light shining on them and so appear as bright points of light darting about in a random or erratic motion. Note that the graphite (or smoke) particles are much larger than the water (or air) molecules. The particles can be seen by the light they scatter but the molecules themselves are too small to be seen.
The irregular movement of the visible particles of graphite (or smoke) is explained as being due to an uneven bombardment of the particles by the invisible molecules of water (or air). It is due to Brownian motion.
We can conclude that the lighter the particles faster the motion and denser the particles slower the motion
(b) Evaporation
The phenomenon of evaporation is associated with the random motion of liquid molecules. At any given temperature, all the molecules do not move with the same velocity. Some move much faster than others. If the fast-moving molecules possess energy greater than the energy with which they are held, they will escape from the surface of the liquid. This phenomenon is called evaporation. The escape of fast-moving molecules lowers the mean kinetic energy of the remaining liquid molecules. This causes a decrease in the temperature of the liquid. That is why evaporation causes cooling.
(c) Diffusion
It is the process by which the molecules of one kind penetrate and intermix with the molecules of another kind.
If a bottle of ammonia is opened in a room, the odor spreads to all parts of the room. This is because the molecules of ammonia escape from the bottle and diffuse into the air molecules.

