Periodic Table:
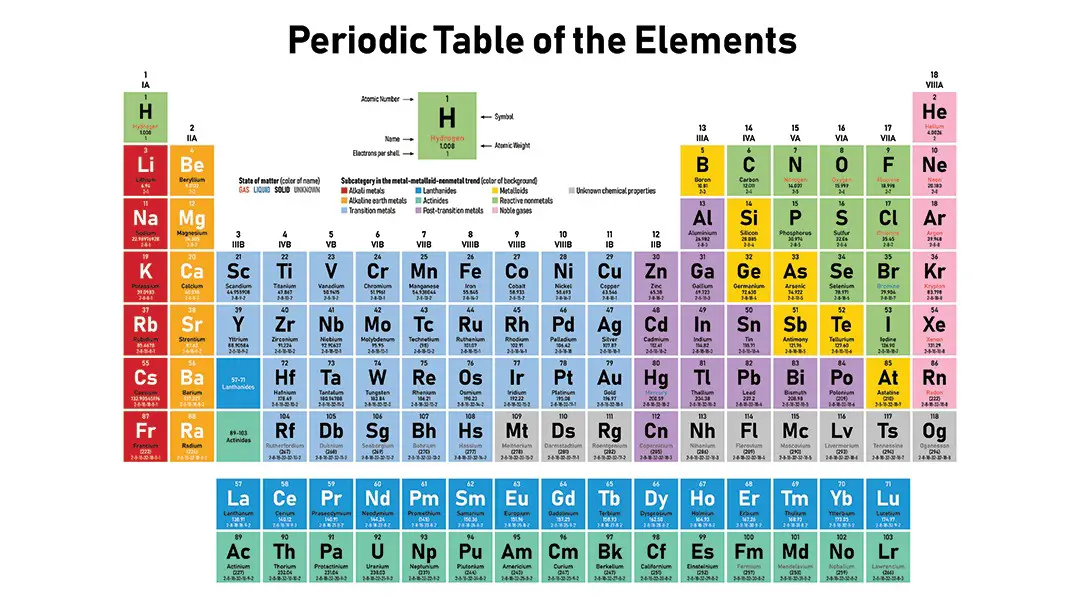
Elements are grouped in order to maximize the atomic number on a Periodic Table where every element possesses one proton greater than the previous element
The table shall be arranged in columns known as 1 – 8 groups and in lines known as periods.
Period: these are the horizontal lines that represent the number of electron shells in an atom
For example, in period 2 elements have two electron shells, in period 3 elements have three electron shells
Group: these are the vertical columns that show the number of external atoms
E.g. Group 4 elements have atoms in the outermost shell with 4 electrons, Group 6 elements have atoms and the exterior shell with 6 electrons.
THE PERIODIC LAW:
The atomic number of the elements’ physical and chemical properties are periodic. Electron arrangements of the elements are listed in the table. Families or classes are called components in the same vertical frame.
Characters in their outer electron shells have identical properties and identical configurations of valence electrons. Elements are called cycles in the same horizontal line.
Periods have the same high level of energy. A map for all the elements is provided in the regular table:
Metals – solids except Hg mercury; good conductivity, bright, malty
Nonmetals — metal gasses or delicate solids — the “stage”
Noble gasses – non-reactive gasses, VIIIA group monoatomic, nearly inert.
The Groups of the Periodic Table
Read also: Organic Chemistry
ALKALI METALS
Alkaline metals are Group 1. These are soft metals with a 1 configuration of the external electron shell. These are the metals that are most involved.
They react with air or water quickly and produce a fundamental solution in water. The 1 electron is missing and +1 positive charged. The 1 electron is included. Please note that if this happens, they have a noble gas electron configuration.
ALKALINE EARTH METALS
Group 2 is earth metals alkaline. We have an s2 configuration on their exterior electron plates. They are more difficult and less reactive than the metals of group 1. They lose their 2-electron ions and are +2-positive.
Also remember that, whenever anything occurs, a noble gas is formed by an electron.
TRANSITION METALS
Groups 3 to 12 contain elements of transformation.
The metals in transition are stronger and less reagent than those in Group 1 & 2. Because their exterior shells occupy the d-orbital, they are often referred to as the d-block elements.
LANTHANIDES
The lanthanide (4f) series have atomic numbers 57-71. These metals are shiny & reactive. Some are used as phosphors that glow when electrons hit them.
ACTINIDES
The actinide (5f) series have atomic numbers 89-103. These metals are all radioactive. Many are manmade. Uranium is important in nuclear energy reactions.
MAIN BLOCK ELEMENTS
Groups 3 to 8 are referred to as main elements of blocks. Aluminum, gallium, indium, nickel, thallium, cherry, bismuth, and polonium are all metals in this group.
Boron, titanium, germanium, arsenic, antimony & tellurium are the metalloids in this category. Hydrogen, oxygen, nitrogen, iron, arsenic, mercury, selenium, fluorine, chlorine, bromine, iodine, and noble gases are the non-metals in this category.
HALOGENS
Group 7 is called the halogens. They form salts with the Group 1 metals.
They are the most reactive nonmetals. Their outer electron shell is p 5 if they gain one electron, they can have the electron configuration of a noble gas. If they do this, they are ions with –1 charge.
CHALCOGENS
Group 6 is called the chalcogens. They have an outer electron configuration of s 2 p 4 so they try to gain 2 electrons so they can have the electron configuration of a noble gas.
If they do this, they become ions with a –2 charge. Oxygen is the most reactive element of this group.
NOBLE GASES
Group 8 is the noble gases. They have filled s and p sublevels in their highest energy level. Having these electron shells filled makes them very stable.
They are not willing to gain, lose or share electrons, so they will not react with other elements.
HYDROGEN
Hydrogen is all in one group. This may either give away an electron or obtain an electron in its 1s1 configuration. Hydrogen can be a metal or non-metal in this respect.
Normally they exchange the electron. It interacts quickly with other molecules or H2 types. On the left side of the bed, it is the only nonmetal.
Periodic patterns in the elements: • atomic range • ionization strength • electron attraction • electron negativity Horizontal & vertical variations may be shown
ATOMIC RADIUS
To find atomic radius, atoms are assumed to be spheres.
The electron cloud size determines the atomic radius for an atom. The radius values are only estimating. These values are measured by finding the distance between 2 nuclei and dividing the distance by 2.
GROUP TREND:
Atomic radius increases as you move from top to bottom in a family. This is because major energy levels (1-7) are being filled with more & more electrons. The electrons get farther & farther from the nucleus.
PERIOD TREND:
Atomic radius generally decreases from left to right as atomic number increases. This is because extra electrons are entering the same level while the nucleus gets larger & more positive.
This draws the electron cloud in towards the nucleus.
Read also: Mole Calculations
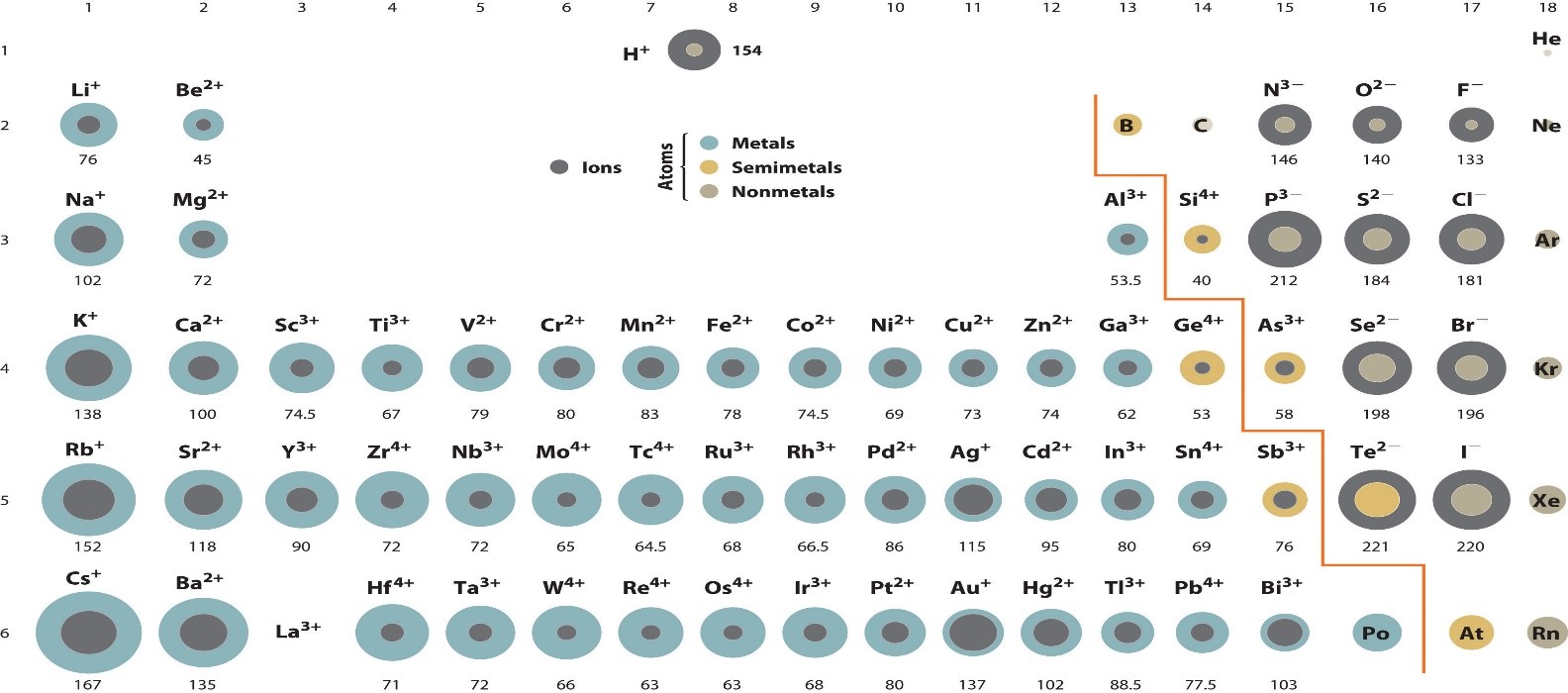
ATOMIC RADIUS OF IONS:
When an atom loses an electron, it has a positive charge. The radius of the atom decreases because there’s a smaller electron cloud. When an atom gains an electron, it has a negative charge. The radius of the atom increases because the electron cloud is larger.
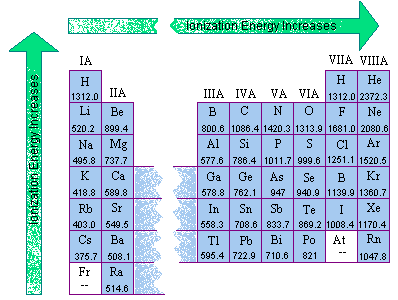
IONIZATION ENERGY
Ionization energy is the energy needed to remove an electron from an atom. GROUP TREND: In vertical groups, ionization energy decreases from top to bottom.
This is because electrons are farther from the nucleus & filled levels cause a shielding effect.
SHIELDING EFFECT:
Inner electrons shield outer electrons from the positive nucleus. This means outer electrons are not held as tightly.
PERIOD TREND:
Ionization energy tends to increase as you move from left to right toward the noble gases. This is because metals tend to lose electrons & nonmetals tend to gain electrons.
All of them want to as stable as the noble gases. [Diagram of Ionization Energy] [Diagram of Electron Affinity]
ELECTRON AFFINITY
Electron affinity is the ability of an atom to attract and hold an extra electron.
GROUP TREND:
Electron affinity decreases from top to bottom of a group. This is because it’s easier for small atoms with the nucleus closer to the outer electrons to gain another electron.
PERIOD TREND:
Electron affinity in a horizontal period increases from left to right. This is because the desire to gain an electron increases the closer you get to fill the energy level. What do you think the electron affinity of the noble gases is? Zero, they are happy like they are.
ELECTRONEGATIVITY
Electronegativity is the measured tendency to attract an electron in a chemical bond. GROUP TREND: Electronegativity decreases from the top to bottom. Smaller atoms have a shorter distance to the nucleus & less shielding effect.
PERIOD TREND:
Electronegativity values increase as you go from left to right.
Metals want to empty their sublevels so they lose electrons. Nonmetals want to gain electrons so they can be like the noble gases.
NOBLE GASES AND TRENDS:
They have the maximum energy in ionization because the electrons don’t want to be killed. That is due to its highly stable packed electron shells. In comparison to other elements, the electron affinity of noble gases is zero. Noble gases have the highest energy of ionization, and null electron concentration and electrons.

