The mineral of salt consists mainly of sodium chloride, a substance that is a chemical compound of a broader class of salts; salt is known as rock salt or halite in its natural form as crystalline minerals.
In sea water, where it’s the main mineral ingredient, salt is contained in huge amounts.
For examples:
- The salt-potassium chloride KCl is then formed when replacing a H in HCl with a potassium atom.
- The diverse and important use of this group of compounds makes Salts an important branch of chemistry
- Such uses include fertilizers, batteries, purifiers, safety products and fungicides.
- Identification of salts
- There are two sections of the salt name.
- The first part is made of the metal or metal oxide used in the reaction carbonate
- The second element is fire.
- By looking at the reactants, the name of salt can be determined.
- Hydrochloric acid also creates chloride finishing salts and Cl – producing chloride ion
- Sodium hydroxide is used to manufacture sodium chloride with hydrochloric acid
- Zinc oxide reacts to zinc sulfate with sulfuric acid.
- Salt storage
- Some salts can be mined, but others must be made in the laboratory.
- When preparing salts, there are two main ideas to consider:
- Was salt shaped soluble in water or is it insoluble?
Read also: Periodic Table & Group Trends
Common salt solubility
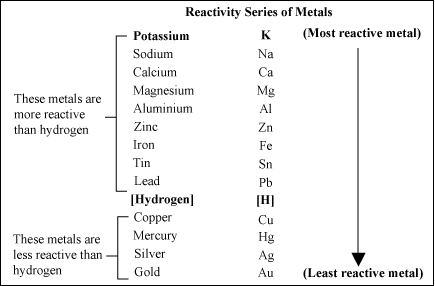
Preparing soluble salts
Method A: adding acid to a solid metal, base or carbonate
preparation of soluble salts
Method:
Add dilute acid into a beaker and heat using a Bunsen burner flame
Add the insoluble metal, base or carbonate, a little at a time, to the warm dilute acid and stir until the base is in excess (i.e. until the base stops disappearing and a suspension of the base forms in the acid)
Filter the mixture into an evaporating basin to remove the excess base
Heat the solution to evaporate water and to make the solution saturated. Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the filtrate in a warm place to dry and crystallize
Decant excess solution and allow crystals to dry
Preparation of pure, Hydrated Copper (II) Sulfate Crystals using Method A
Acid = Dilute Sulfuric Acid
Insoluble base = Copper (II) Oxide
Method:
Add dilute sulfuric acid into a beaker and heat using a Bunsen burner flame
Add copper (II) oxide (insoluble base), a little at a time to the warm dilute sulfuric acid and stir until the copper (II) oxide is in excess (stops disappearing)
Filter the mixture into an evaporating basin to remove the excess copper (II) oxide
Leave the filtrate in a warm place to dry and crystallize
Decant excess solution
Blot crystals dry
Equation of reaction:
Copper (II) Oxide + Dilute Sulfuric Acid → Copper (II) Sulphate + Water
CuO (s) + H2SO4 (aq) → CuSO4 (s) + H2O (l)
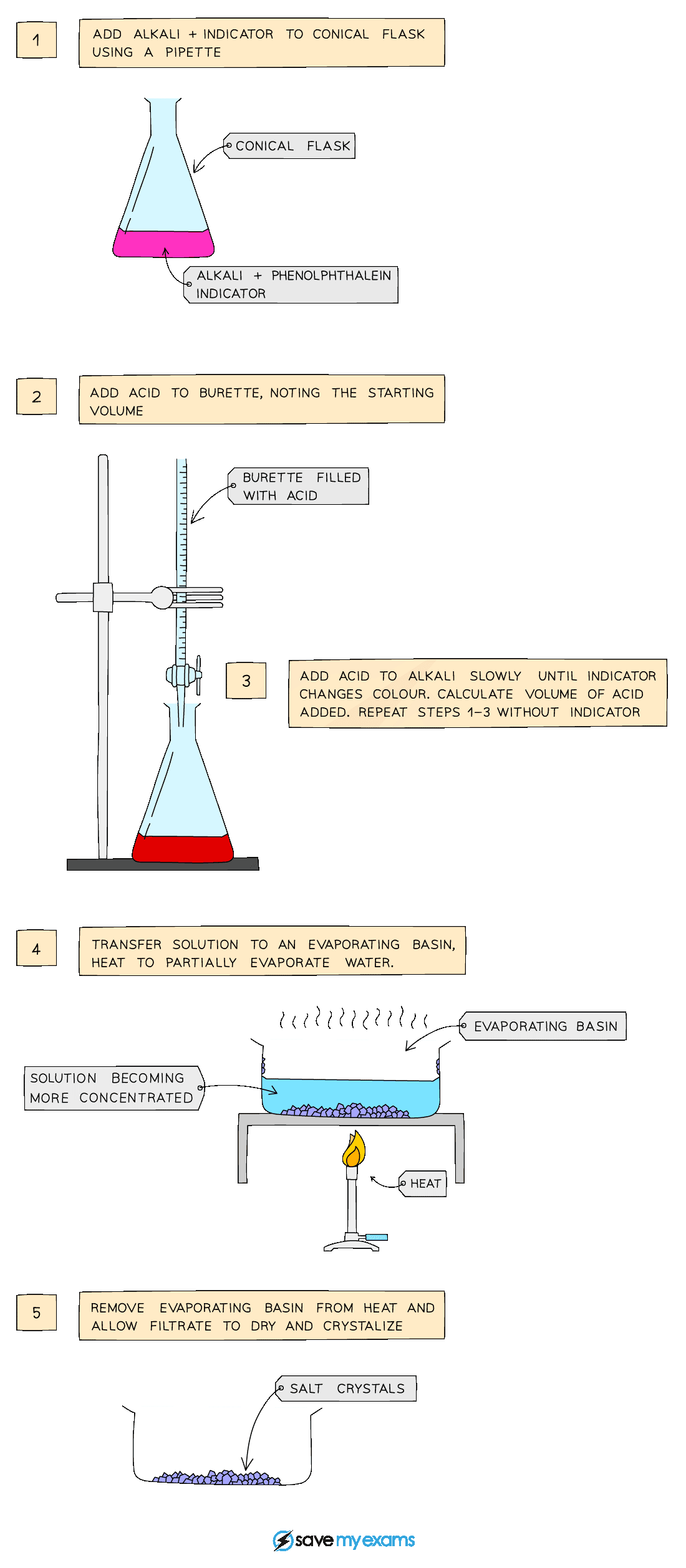
Method B: reacting a dilute acid and alkali
Diagram showing the apparatus needed to prepare a salt by titration
Read also: Organic Chemistry
Method:
Use a pipette to measure the alkali into a conical flask and add a few drops of indicator (phenolphthalein or methyl orange)
Add the acid into the burette and note the starting volume
Add the acid very slowly from the burette to the conical flask until the indicator changes to appropriate colour
Note and record the final volume of acid in burette and calculate the volume of acid added (starting volume of acid – final volume of acid)
Add this same volume of acid into the same volume of alkali without the indicator
Heat to partially evaporate, leaving a saturated solution
Leave to crystallise decant excess solution and allow crystals to dry
Extended Only
Preparing Insoluble Salts
Insoluble salts can be prepared using a precipitation reaction
The solid salt obtained is the precipitate, thus in order to successfully use this method the solid salt being formed must be insoluble in water
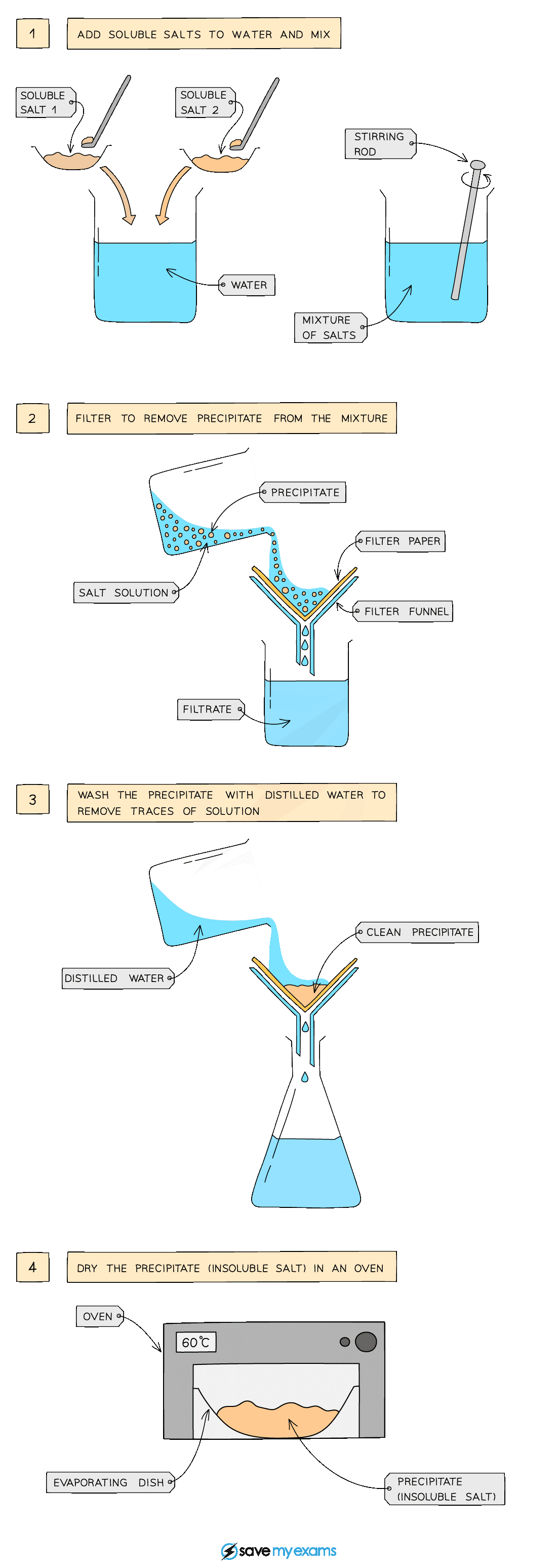
Using two soluble reactants
Diagram showing the filtration of the mixture to remove the precipitate
Method:
Dissolve soluble salts in water and mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash filtrate with water to remove traces of other solutions
Leave in an oven to dry
Preparation of Pure, Dry Lead (II) Sulfate Crystals using a precipitation reaction
Soluble Salt 1 = Lead (II) Nitrate
Soluble Salt 2 = Potassium Sulfate
Method:
Dissolve Lead (II) Nitrate and Potassium Sulfate in water and mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash precipitate with water to remove traces of potassium nitrate solution
Leave in an oven to dry
Equation of reaction:
Lead (II) Nitrate + Potassium Sulfate → Lead (II) Sulfate + Potassium Nitrate
Pb(NO3)2 (s) + K2SO4 (s) → PbSO4 (s) + 2KNO3 (s)

