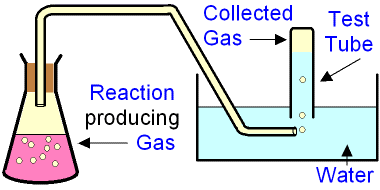
Collection of Gases
Speed of Chemical Reactions:
When a gas is released, the speed of the reaction can be determined when the volume of the gas is reported over a given time interval that can be calculated by a gas syringe, or when the gas is expelled into the air, which can be measured through a meter.
The following features should be taken into consideration when extracting a gas:
- The air-related density.
- its water solubility.
The system of the upstream air transport in which the air is transferred should be used to absorb the gas (that is to be collected) is more volatile than air.
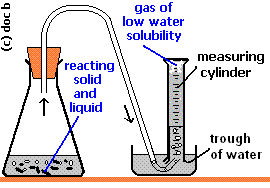
If the gas is lighter than air, it can be collected using the upward delivery method, in which the air is displaced downward, and the gas is collected in an upside-down container.
For gases that are insoluble or slightly soluble in water, the method of displacement of water may be used, in which the gas is passed through water into an upside-down container; the gas collects on top and the water gets displaced downwards.
Read also: Energy Changes
Measurements:
The study of chemistry requires the measurement of various quantities, such as mass and volume.
Mass:
The quantity of matter it contains is the density of a substance. The measurement is one gram (g) in small amounts or one kilogram (kg) in greater quantities. The stability of the beam or electrical balance is calculated.
Electronic balance.
Time:

The S.I unit is seconds (s) for the time. Minutes (min) and hours (h) are other units. For time measurement, a stopwatch or a stopped clock is used.
Temperature:
The S.I word for temp is Kelvin (K). A third level is the degree Celsius (° C).
A thermometer should be used to determine the temperature. Mercury for glass and alcohol in the glass is commonly used for experiments with temperatures from-10 to 110 ° C.
Read also: Elements, Compounds & Mixtures
Volume:

The volume of space a material occupies is its length. This is expressed in cm3, dm3, or liters (L). It is expressed in cm3. The volume of a fluid is measured using many laboratory devices.
Some of these are as follows:
A beaker is used to measure approximate volumes.

A measuring cylinder is accurate to the nearest cm³.
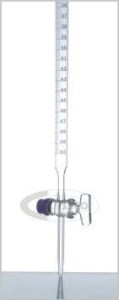
A burette is accurate to the nearest 0.1 cm³. The volume of liquid required is runoff from the bottom through a tap.
The pipette measures fixed volumes (25cm³, 50cm³, 10cm³, etc.) very accurately.