Qualitative analysis in chemistry is the determination of a sample’s chemical composition. It contains a collection of analytical methods for chemistry that include non-numeric details on a specimen.
Qualitative analysis may tell you whether a sample contains or does not include an atom, ion, functional group, or compound but does not provide data on its quantity.
In contrast, a quantitative analysis is called the quantification of a sample.
Techniques and Tests
Qualitative testing includes chemical tests, such as a blood test for Kastle-Meyer and a starch iodine test. The flame test is also a common qualitative test used in inorganic chemical analysis.
Typical measurements of qualitative analysis include a change from color, melting point, odor, reactivity, radioactivity, boiling point, and precipitation. Distillation, extraction, freezing, coloration, and spectroscopy include methods.
Branches of Qualitative Analysis
Organic qualitative analysis (such as iodine test) and inorganic qualitative analysis (such as flam test) are two main areas of qualitative analysis.
An inorganic analysis investigates the chemical and ionic structure of a sample, usually in aqueous solution, through observation of ions.
organic analysis tends to look at molecular types, functional groups, and chemical connections.
Example
She used qualitative analysis to find that the solution contained Cu2+ and Cl– ions.
Read also: Rate of Reaction
Flame Tests
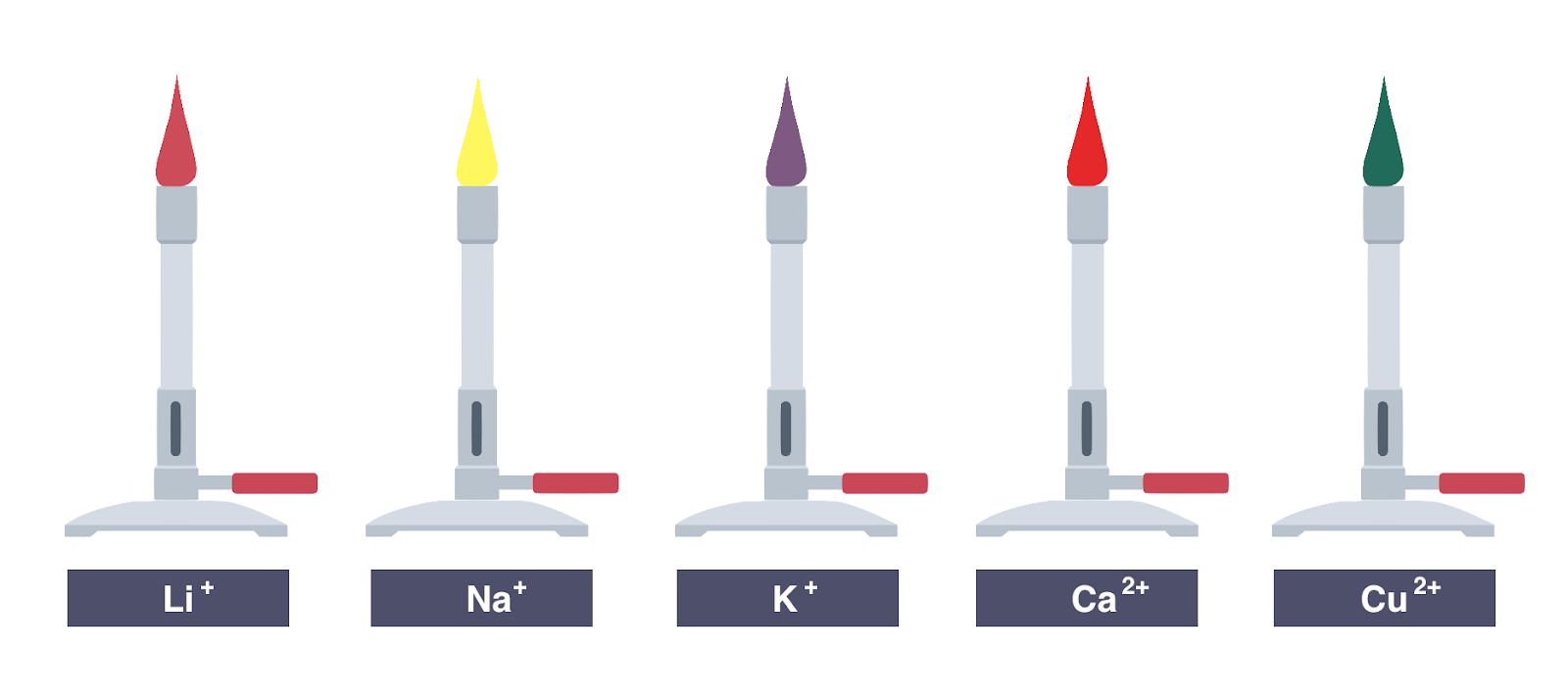
- The flame test is utilized by the color of the flame it produces to identify metal cations.
- The unreactive metal wire such as nichrome or platinum is fed a small sample of the compound.
- The flaming color is observed and used in metal identification.
Results
- Red = Li+
- Yellow = Na+
- Lilac = K+
- Orange-red = Ca2+
- Blue-green = Cu2+
- Bright white = Mg2+
Flaming test sources of error
- A color in bright flames may be difficult to determine.
- Metal ion impurities can alter the efficiency.
- A high concentration of metal ions is necessary for the solution.
Test for Anions
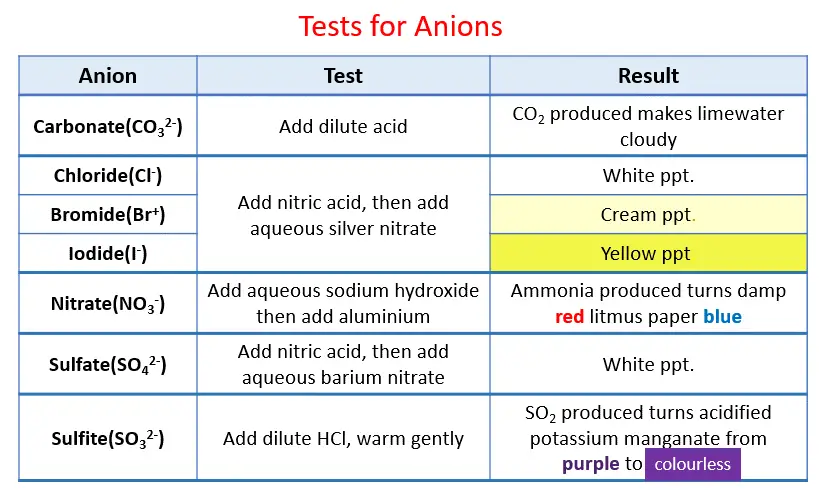
The silver halide falls.
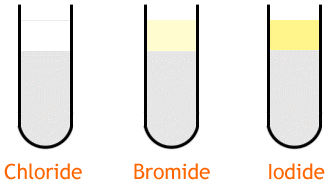
In practical tests, a common error is to confuse silver halides as the color differences between them are not sharp.
The colors are as follows:
o Chloride ions provide a white silver chloride precipitation
o Bromide ions give silver bromide cream precipitation
iodide ions give silver iodide yellow precipitate
The colors formed by precipitates of the silver halides
Read also: Preparation of Salts
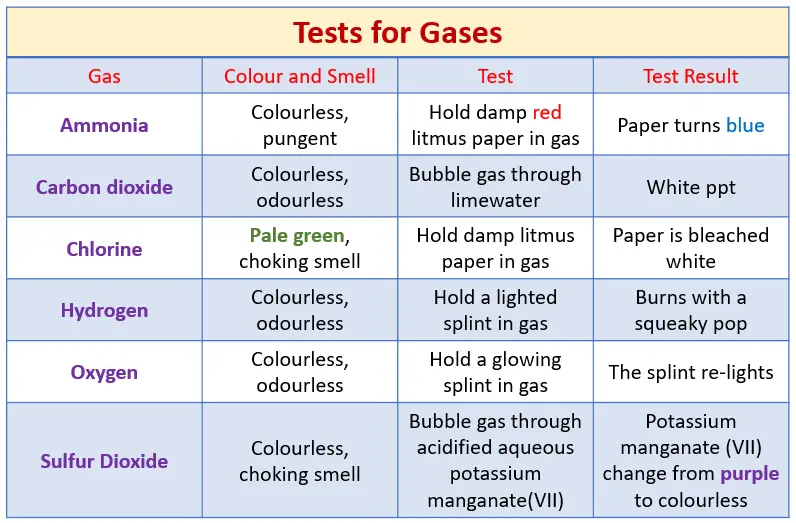
Tests for Gases