DEFINITION
The kinetic matter (particle hypothesis) hypothesis states that all matter contains very, very small, continually moving particles or continuously moving.
The amount of energy they have and their interaction with them and other particles decide the degree at which they travel.
EXPLANATION
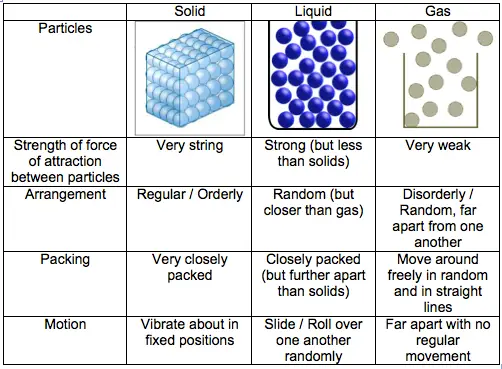
The theory of kinetic particles describes the properties of various material states. Particles are found in solids, liquids, and gasses. The structure is different and they travel in various directions.
The table outlines the structure and movement of the solids, liquids, and gasses of the particles and provides simple diagrams for their structure.
Read also: Experimental Techniques
SOLIDS
| Properties | Reason |
| They have a fixed shape and cannot flow. | The particles are free to move around each other. |
| They cannot be compressed or squashed | The particles are close together and have no space to move into |
LIQUIDS
| Properties | Reason |
| They flow and take the shape of their container | The particles are free to move around each other |
| They cannot be compressed or solid | The Particles are close together and have no space to move into |
Gases
| Properties | Reason |
| They flow and fill their container | The particles are free to move from one place to others because of their shape of the container |
| They can be compressed or solid | As the particle are far apart and have space between them so they can move. |
Read also: Balancing Chemical Equations
THERMAL EXPANSION OD SOLID, LIQUID, GAS
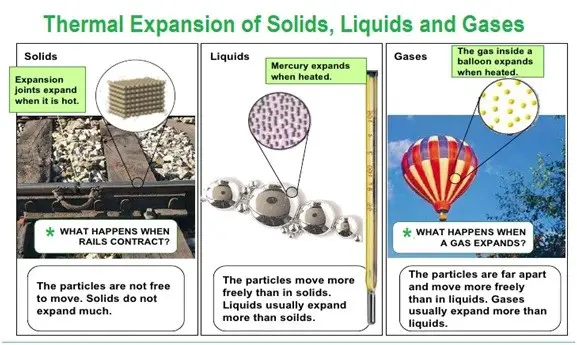
When warm, all three states of matter (solid, liquid, and gas) expand. The atoms are not expanding, they instead of using up space.
The atoms of a solid vibrate faster at its fixed points if it is hot. Therefore, the relative rise in solid size is minimal when heated.
The metal tracks have small holes, meaning that the rails extend through these holes as the sun heats them and doesn’t crumble.
Liquids are expanding for the same purpose, but the connections between different molecules are usually less strong than solids.
It is the concept behind the thermometers of fluid in the water. The temperature increases and the liquid expands and hence the glass reduces.
Gas molecules are farther apart and drawn to each other weakly. Energy helps the molecules travel quicker, which ensures that a gas’s volume increases above a solid or liquid’s pressure, (heat energy transforms to kinetic energy).
Gasses trapped in a set volume will therefore never extend, which leads to an increase in heat as the temperature changes.

