What is Mole?
The mole (abbreviated mol) is the SI measurement of the quantity of a ‘chemical object.’ The sum of a material containing as many particles as atoms in 12 g pure carbon-12 is described. Thus, 1 mol contains 6,022 bis1023 basic substance entities.
Read also: METALS & REACTIVITY SERIES
Formulas
Avogadro’s Number:
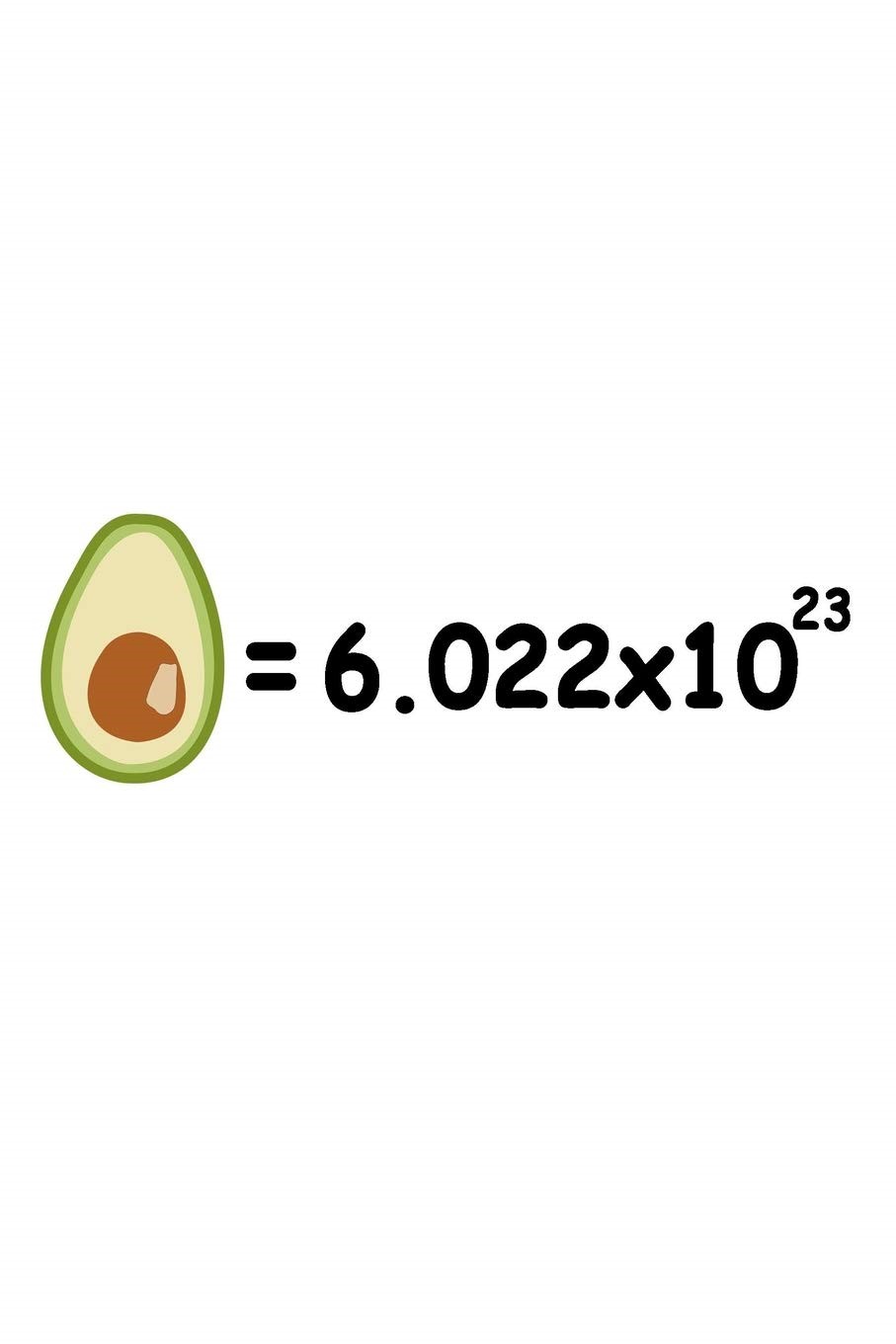
Amadeo Avogadro initially suggested that gas volumes are proportionate to the number of atoms or molecules, at certain pressures and temperatures, regardless of gas type.
And if the precise proportion has not been calculated, the principle is acknowledged. The number of Avogadro is a proportion which, on an atomic scale, links molar mass to human physical mass.
The number of Avogadro is known as the number of elementary particles per mole of a substance (molecules, atoms, compounds, etc.) This is equivalent to 6.022 to 1023 mol-1 and is the symbol NA.
The number of Avogadro is like a dozen or a gross term. Twelve molecules are a dozen. There are 144 molecules in the average. The amount of Avogadro’s molecules is 6.022 in 1023.
Researchers may analyze and match significant numbers with the amount of Avogadro, which is helpful because liquids contain an immense quantity of atoms and molecules at regular amounts.
Read also: KINETIC PARTICLE THEORY


