What Is Organic Chemistry?
Organic chemistry involves the study of structure, properties, composition, and reaction, and preparation of carbon-containing compounds, including, inter alia, hyDOC, ammonia, hydrogen, nitrogen, oxygen, halogens, phosphorus, silicon, and sweat, which include most compounds at least one carbon-hydrogen bond.
Originally, until extended to include human substances like plastics, this branch of chemistry was restricted to materials made by living beings.
There are extensive uses of organic compounds and medicinal, petrochemical, medicine, explosives, paints, and cosmetics are included but they are not limited to them.
Read also: Mole Calculations
Where Is Organic Chemistry Used?
Organic chemistry is a very experimental process where chemical chemists build new structures and investigate the properties of compounds already existing. For ACS chemists and Ph.D. pharmacists, this is the most popular field of research.
All around us are organic compounds. It is vital to the economic development in the rubber, coal, drugs, cosmetics, detergents, coatings, teething and agricultural sectors, to list only a handful.
Organic compounds and their role in life cycles draw on the very basis of biochemistry, biotechnology, and medicine. At least partly organic compounds are present in many modern high-tech materials.
Most of the time organic chemists spend making new compounds and finding effective ways to synthesize previously established compounds.
Biotechnology
Biotechnology (“biotech” for short) is an engineering field in which products are created for a specific purpose by the use of live organisms and bioprocesses.
Plant cultivation was recognized as the oldest example of genetics and the predecessor to contemporary genetic engineering and technologies for cell and tissue culture. Organic chemistry appears in almost all biotechnology products.
In health care, seed production and forestry, non-food seed use and other products (for example, biodegradable plastics, palm oil, biofuels), and environmental applications, biotechnical technology is used.
Names of organic compounds:
All organic compounds are named using a system based on the structure of the molecule. The first part of the name gives you the number of carbon atoms in the chain.
1C =meth
2C =eth
3C =prop
4C =but
The second part of the name gives you the type of compound it is and enables you to predict how it will react (the functional group)
-ane = alkane
-ene =alkene
-anol = alcohol
-anoic acid =carboxylic acid
e.g.
Methane Ethane
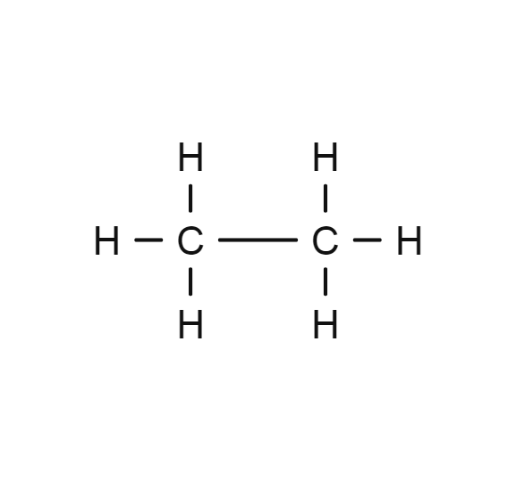
Fuels
Hydrocarbons are excellent fuels (organic carbon molecules only and hydrogen). To obtain carbon dioxide and water they can burn air with oxygen.
The reaction produces a lot of thermal energy; the reaction is very exothermic. Because of carbon dioxide, minimal oxygen carbon monoxide can be formed. The carbon monoxide is very toxic, and that is dangerous.
Examples of fossil fuels are coal derived from petroleum (crude oil). For millions of years, fossil fuels have been extracted from decaying plants and animals buried beneath several layers.
It takes so much time to produce fossil fuel, and it can’t be replaced as it is used. Fossil sources are examples:
Carbon a solid power. Electricity can be produced by burning in power stations. Oil fuels like petrol, diesel, and kerosene are liquid fuels. Such are for the use of vehicles: buses, lorries, and aircraft Natural fossil (methane) fossil fuel can also be used in power plants in cooking homes for energy generation.
We can burn very easily when solid fuel is in a powdered state. Coal dust can lead to explosions in mines.
Dust can have the same impact on grain stores. Methane can be produced from plant and animal waste.
This is sustainable and is referred to as biogas.
Read also: METALS & REACTIVITY SERIES
Petrochemicals
Petroleum is a mix of hydrocarbons (carbon and hydrogen compounds only). A fractional distillation column may be added to the hydrocarbons in crude oil. The oil is hot, the various hydrocarbons are gases and the pillars go up.
Those with low boiling points can rise more until they become a liquid. The shorter the molecule of the hydrocarbon the greater the boiling point. A fraction is called every group of compounds. The fractions are shown in the diagram:
Homologous series
A series of organic compounds with the same general formula and chemical properties is a series of homologous ones. They are responding the same way as they have close bonds.
Their physical characteristics show a progressive change when the carbon chain is longer:
- Increased boiling point
- They are less flame-retardant (not as quickly set fire)
- They’re becoming more viscous (not easily)
- They become less volatile (not as easily evaporated) All of the organic compounds are composed of molecules that are bound together. Soft forces such as van der Waals bind the molecules together.
Alkanes
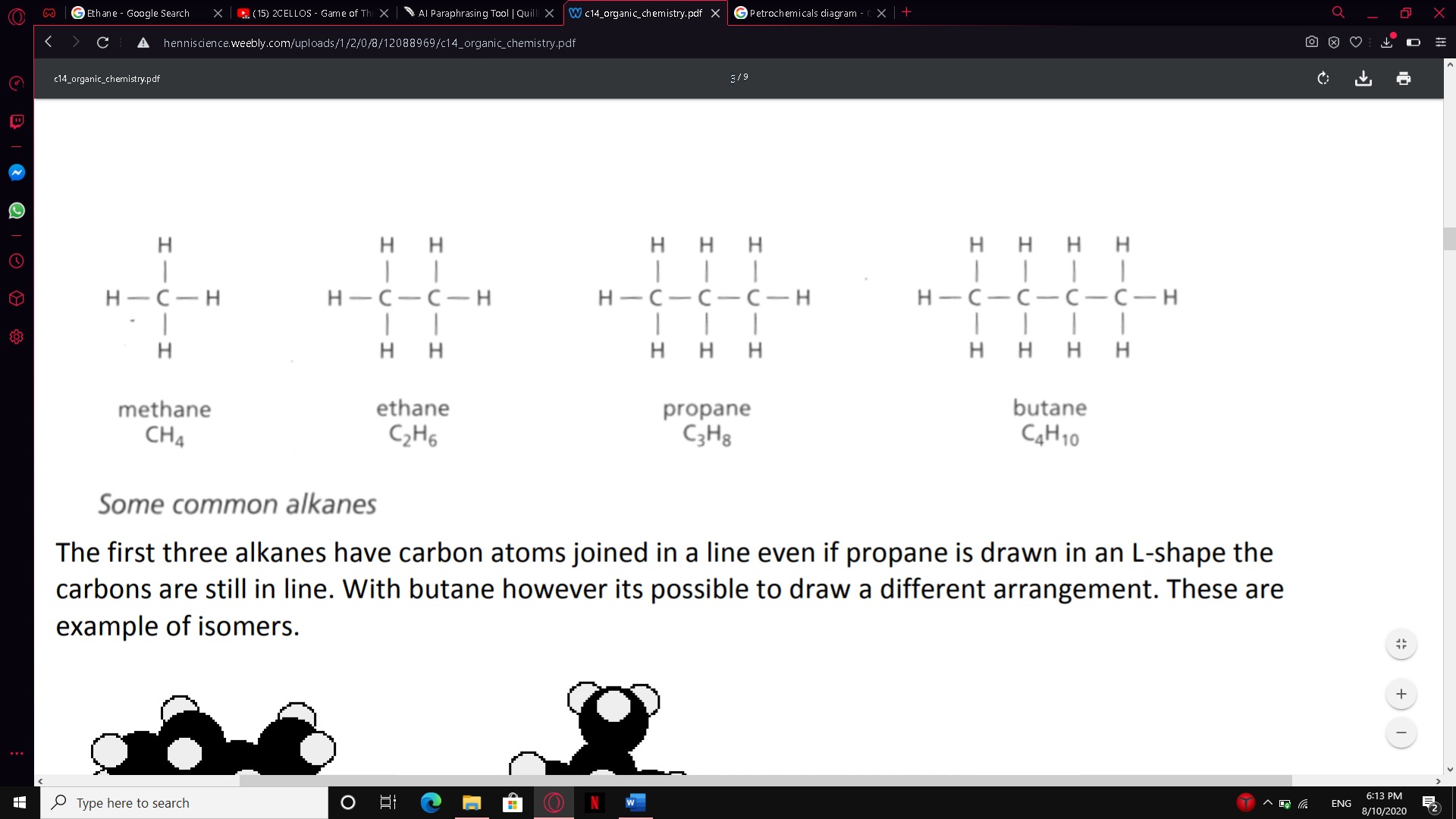
Alkanes are the same as the general composition CnH2n + 2. This is a hydrocarbon and is primarily present in raw crude. They can be described by molecular formulae or graphic formulae as other organic compounds.
Alkanes are hydrocarbons that are saturated because there are just bonds between carbon and carbon. The number of carbon and hydrogen atoms in a molecule is seen in a chemical formulation.
Ethane C2H6, for example. A graphic diagram shows how the atoms, for example, ethane H3C-CH3, are currently mixing. Below shows some common alkanes and their molecular and graphical formulas:
The first three alkanes have carbon atoms joined in a line even if propane is drawn in an L-
The first three alkanes have carbon atoms joined in a line even if propane is drawn in an L-shape the carbons are still in line. With butane however its possible to draw a different arrangement. These are examples of isomers.
Isomers
Isomers have the same molecular formula but different structural formulas.
They behave similarly chemically but their physical properties can be different. Generally, the more branched the molecule the lower the boiling point.
Chemical properties of alkanes
They are generally unreactive however they do burn exothermically making them good fuels. For example, methane is the main part of natural gas. All alkanes form carbon dioxide and water when they are burned in a plentiful supply of air.
If the oxygen is in short supply carbon monoxide and soot (carbon) can be formed.
Methane + oxygen-carbon dioxide + water
CH4 (g) + 2O2 (g) → CO2 (g) + H2O (g)
The only other reaction of alkanes is with chlorine. In the presence of sunlight one of the hydrogens in the alkane can be replaced by a chlorine atom this is called a substitution reaction.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Chloroethane and hydrogen chloride are formed.
The reaction doesn’t stop there if there is plenty of chlorine it can continue:
- CH3Cl + Cl2→ CH2Cl2 + HCl
- CH2Cl2 + Cl2→ CHCl3 + HCl
- CHCl3 + Cl2→ CCl4 + HCl
Alkenes
Alkenes are unsaturated carbohydrates and have in the molecule at least one double carbon bond. Ethane and Propene are examples of alkenes shown here: Alkenes are combusted in the same manner as alkanes, but they are also subject to a type of reaction called an addition. It makes them explosive rather than alkanes.
The double bond is often broken and used to connect with someone else. Bromine, hydrogen, water, and itself are common additional reactions.
Addition reactions with bromine
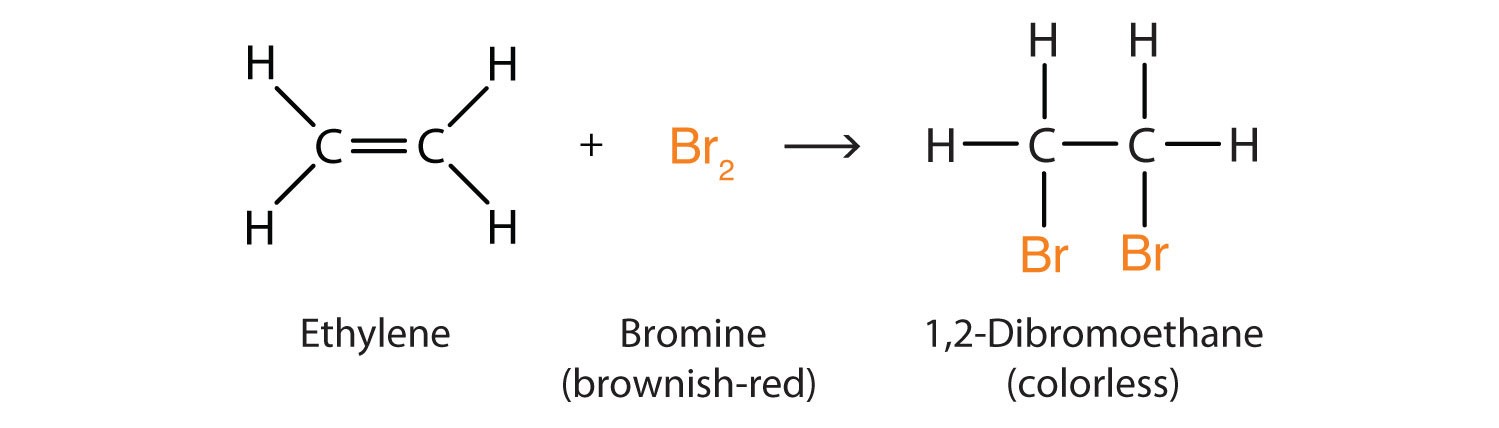
When alkenes are added to bromine water the bromine molecule adds to the alkene forming a bromoalkane. The bromine water turns from orange to colorless.
As saturated compounds do not react with bromine water so bromine water is used to test for unsaturation (double bonds).


