What is the Rate of Reaction?

For chemistry, the reaction rate is the level at which a chemical reaction takes place. The concentration of a compound produced in a unit of time (amount per unit volume) or the concentration of a reactant absorbed within one unit of time is also expressed. Additionally, the number of reactants absorbed and products produced in a single unit of time may be described.
Suppose, for example, that the chemical equation is in balance with a reaction.
A + 3B + 2Z area.
The rate can be presented as an alternative:
d[Z]/dt, – d[A]/dt, – d[B]/dt, dz / dt.
The concentration of t is the time of [A], [B], and [Z], and a, b and z are the concentrations of these compounds. Keep in mind that all these six phrases vary but are related.
Read also: Qualitative Analysis
Collision Theory
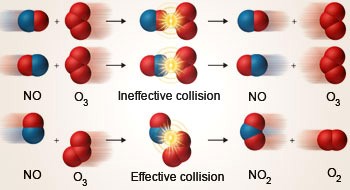
The theory of collision used to predict the rates and especially gases of chemical reactions. The theory of collisions is based on the premise that it is appropriate to carry or collide between the reaction species (atoms or molecules).
Nevertheless, not every collision causes chemical transition. A collision is only efficient in generating chemical transition if the combined species has a minimum internal energy content, equal to the reaction initiation energy.
In a way conducive to the required rearrangement of atoms and electrons, the colliding species should be oriented. The rate at which a chemical reaction takes place is equal to the rate of successful collisions, thus, according to the collision principle.
Since it is possible to determine atomic or molecular collision frequencies with a certain degree of accuracy for gas (with the implementation of cinematic theories) only, the implementation of the principle of collision is limited to gas-phase reactions.
Factors Affecting the Rate of Reaction
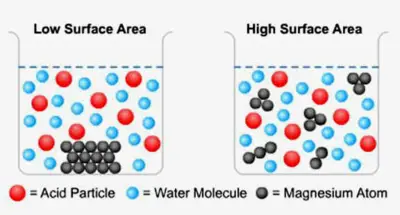
Effect of surface area
- The larger the reactant particle size, the higher the reaction rate.
- This is as the area of the reactant object subjected to the collision is higher, the probability of the collision decreases as much as the number of successful collisions. More compounds are produced per unit time and the reaction rate increases accordingly
For example, the reaction between hydrochloric acid and marble chips (calcium carbonate) is as follow:
HCl(aq) + CaCO3(s) → CaCl2(aq) + CO2(g) + H2O(l)
The rate of reaction can be found by measuring the volume of CO2 gas given out per unit time. The volume of CO2 is determined at regular intervals. The setup of the apparatus is as follow:
- It is found that powdered marble chips react faster than big lumps of marble chips
- This is because powdered marble chips are smaller in size and hence have a larger total surface area
Effect of concentration
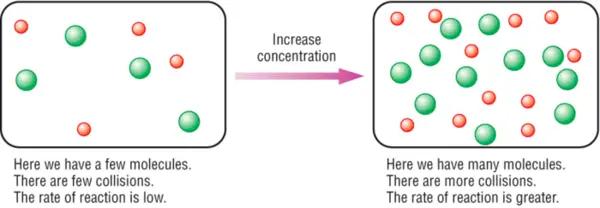
- The greater the reactant particle size, the higher the reaction rate.
- Since there are more reactant particles per unit volume in a more condensed solution. The collision frequency of the reactant particles is increased and the successful collision frequency is therefore increased too. There are more goods per unit time and thus the reaction rate is higher.
- For examples, magnesium bead-hydrochloric acid reaction will be as follows:
Zn(s) + HCl(aq) + H2(g) ZnCl2(aq)
The reaction rate can be measured by calculating the time taken to vanish from the sight of the magnesium ribbon.
The time taken for the magnesium rope to dissolve is shortened when HCl occurs at a higher concentration.
- The more time, the greater the rate of reaction, the more the reaction rate becomes inversely proportional to the time.
- Often increased concentration still does not increase the reaction rate:
Reactions involving only a single reactant particle splitting
- The reaction rate does not depend on the colliding rate.
- The concentration of the reactant particles is determined by the sum of energy.
- When a large portion of particles has more energy than the activation energy, the rate of reaction is decreased and more particles are separated per unit of time.
ii. Where a catalyst is working as fast as it can
- If the reactant particle has a large enough concentration and a minimum catalyst number, the reaction continues
- If the reactant particle size is raised as the catalyst is exhausted, the reaction rate does not improve.
- The reaction rate can be improved by adding additional catalysts
Read also: Purification and Separation
Effect of pressure
- The higher the system load, the higher the reaction rate
- The reason for concentration is the same as that of the result. Increased pressure can, however, only increase the reaction time of gases. This has little effect on change in reaction pressure that either includes solid or liquid.
Effect of temperature
- The higher the reaction system temperature, the higher the response time.
- Since the total kinetic energy of the particles increases at higher temperatures, so:
- There is more energy than or equal to the activation of the responding particles (this is the major cause)
- The probability of interaction of the interacting particles moving at higher speed increases.
- This raises the likelihood of a good collision. There is a higher rate of reaction with more materials per unit time.

