METALS & REACTIVITY SERIES
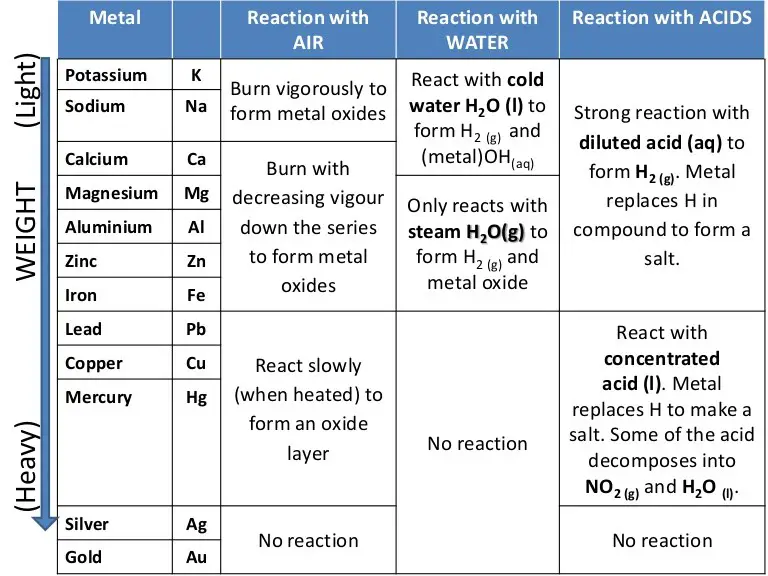
- The chemistry of the metals is studied by analyzing their reactions with water, dilute acid and air.
- Based on these reactions a reactivity series of metals can be produced
- The series can be used to place a group of metals in order of reactivitybased on the observations of their reactions with water, acid and air.
Carbon and the reactivity series mnemonic
Carbon is an important element and has its own place on the reactivity series
Its use in the extraction of metals from their oxides is discussed in this section but a more complete reactivity series with an accompanying mnemonic to help your memories it is below
The reactivity series mnemonic
“Please send lions, cats, monkeys and cute zebras into hot countries signed Gordon”
Reactions with Aqueous Ions & Oxides
The reactivity of metals increases going up the reactivity series
This means that a more reactive metal can displace a less reactive metal from its oxide by heating
Example: Copper(II) Oxide
It is possible to reduce copper(II) oxide by heating it with magnesium
As magnesium is above copper in the reactivity series, magnesium is more reactive so can displace copper
The reducing agent in the reaction is magnesium:
CuO (s) + Mg (s) → Cu (s) + MgO (s)
Read also: Experimental Techniques
Other common reactions
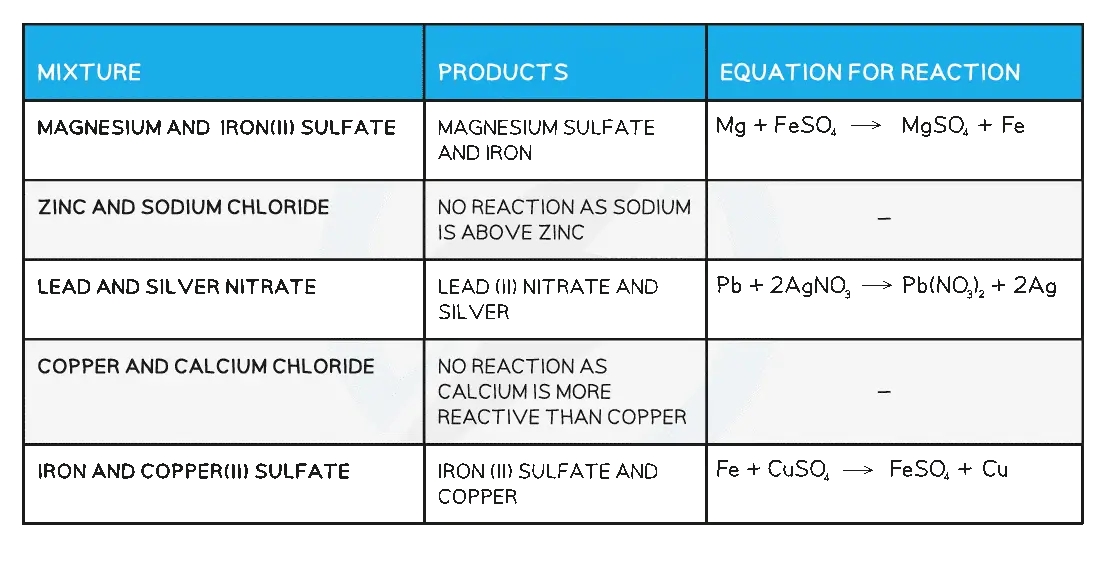
Displacement reactions between metals and aqueous solutions of metal salts
Any metal will displace another metal that is below it in the reactivity series from a solution of one of its salts
This is because more reactive metals lose electrons and form ions more readily than less reactive metals, making them better reducing agents
The less reactive metal is a better electron acceptor than the more reactive metal, thus the less reactive metal is reduced. (OIL-RIG: reduction is gain of electrons)
Example: Zinc and copper(II) sulfate
As Zinc is above copper in the reactivity series, zinc is more reactive so can displace copper from copper(II) sulfate solution:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Other Common Reactions
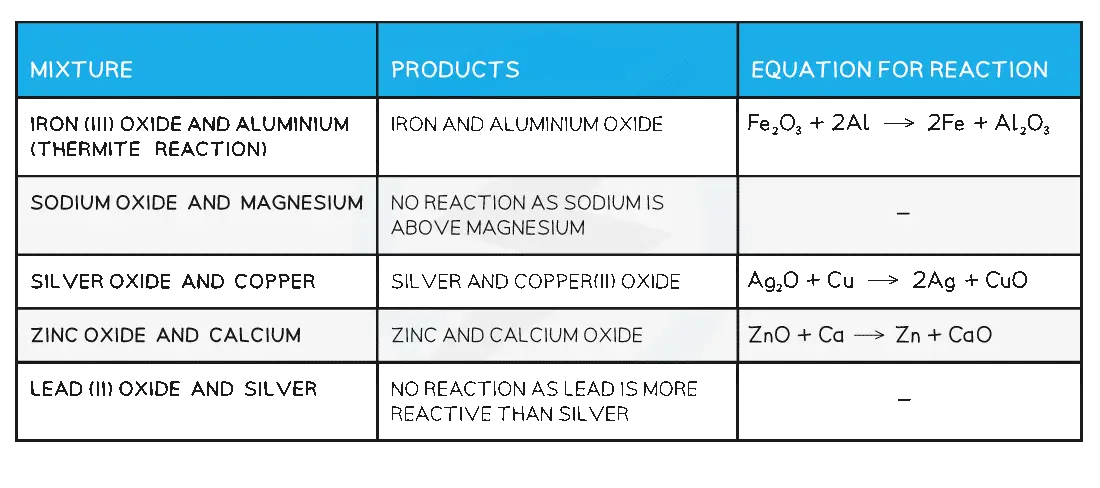
Extended Only
Heating Metal Hydroxides, Carbonates & Nitrates
Thermal decomposition reactions
Some compounds decompose or breakdown when they are heated to sufficiently high temperatures
These reactions are called thermal decomposition reactions
A common example is the thermal decomposition of calcium carbonate (limestone), which occurs at temperatures above 800ºC:
CaCO3 → CaO + CO2
Thermal decomposition of metal hydroxides
Most metal hydroxides undergo thermal decomposition
Water and the corresponding metal oxide are the products formed, for example zinc hydroxide thermally decomposes as follows:
Zn(OH)2 → ZnO + H2O
Group II metal hydroxides decompose similarly but the Group I hydroxides (apart from lithium) do not decompose due to their having a higher thermal stability
Thermal decomposition of metal carbonates
Most of the metal carbonates and hydrogen carbonates undergo thermal decomposition
The metal oxide and carbon dioxide are the products formed, for example magnesium carbonate thermally decomposes as follows:
MgCO3 → MgO + CO2
Group I carbonates (again apart from lithium carbonate) do not decompose when heated
This is due to the high thermal stability of reactive metals; the more reactive the metal then the more difficult it is to decompose its carbonate
CuCO3 for example is relatively easy to thermally decompose but K2CO3 does not decompose.
Thermal decomposition of metal nitrates
All of the metal nitrates decompose when they are heated
Group I nitrates decompose forming the metal nitrite and oxygen, for example sodium nitrate decomposes as follows:
2NaNO3 → 2NaNO2 + O2
Most other metal nitrates form the corresponding metal oxide, nitrogen dioxide and oxygen when heated, for example copper nitrate:
2Cu(NO3)2→ 2CuO + 4NO2 + O2
Read also: Energy Changes
Aluminum and its apparent lack of reactivity
Aluminum is a curious metal in terms of its reactivity
It is placed high on the reactivity series but it doesn´t react with water or acids
This is because the surface of aluminum metal reacts with oxygen in the air forming a protective coating of aluminum oxide:
4Al + 3O2 → 2Al2O3
The aluminum oxide layer is tough, unreactive, and resistant to corrosion
It adheres very strongly to the aluminum surface and protects it from reaction with other substances, hence making it appear unreactive

