What are the Macro Molecules?
A relatively smaller molecule or a low molecular weight molecule that can regulate a biological system supplement in biology a molecule is relatively small and, as opposed to a macromolecule. The molecule has a relatively low size and weight.
Polymerization is called a method of merging smaller units into a large polymer macromolecule.
Monomer:
Small molecules, that join together to form one large polymer molecules.
There are two kinds of polymer:
- Synthetic Polymer
- Natural Polymers
Synthetic Polymers:
Synthetic Polymers are manmade polymers that are formed by
Addition Polymerization – Addition Polymers
Condensation Polymers – Condensation Polymers
Read also: Elements, Compounds & Mixtures
Addition Polymers:
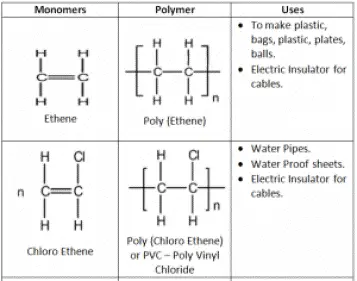
Addition Polymers are made from unsaturated monomers through an addition reaction.
The simplest part of the polymer which is repeated many times to form the polymer.
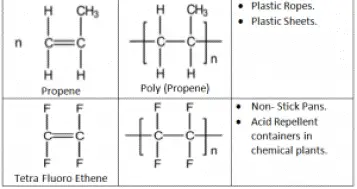
Also, Polymerization of an alkene is converted into Poly alkene.
Alkane is converted to Alkane.
Making of Poly Ethene conditions:
Temperature – 200 °C
Atmospheric Pressure: 1000
Catalyst – Zeigler’s Catalyst – TiCl4 + (C2H5)3 Al – Mixture of Titanium Tertre Chloride and Tetra Aluminum
Perspex – Used in making car windows
Teflon – Used in production of utensils, raincoats, and rubber tubing’s
Polystyrene – Disposable glasses and plastics.
Need Help? Ask Our Chat Assistant!
Condensation Polymers:
When two different monomers join, each with two functional groups. The monomers join their functional groups, by getting rid of or eliminating small molecules.
Condensation Polymers are made from monomers containing alcohol, acid, or amino functional groups.
Through the elimination of small molecules like H2O. E.g. Nylon and Terylene.
Nylon/ Polyamide:
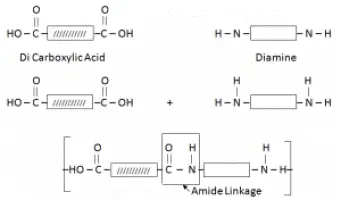
It is formed by the combination of Di Carboxylic Acid and Di Amine.
Why Nylon also called Poly Amide – due to the presence of Amide Linkage.
Uses of Nylon:
Nylon is used in making synthetic fibers
In the production of ropes, fishing lines, and clothes
For making raincoats and parachutes.
Terylene / Polyester:
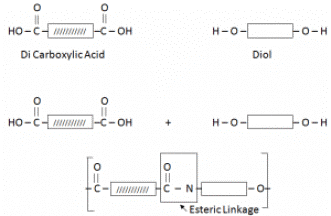
Terylene is formed by the combination of Dicarboxylic Acid and Diol (Hydroxyl Group).
Uses of Terylene:
- Fabrics – That are strong, resists stretching sinking. Doesn’t crumple.
- Sleeping bags are made using Terylene. Hence, they do not shrink (Synthetic Fibre).
Disadvantages of Plastic:
Plastics are non-biodegradable, can’t be decomposed by bacteria. Hence, they result in polluting the earth.
Produce toxic gases (HCl) when burnt, this contributes to acid rain.
Plastics are Carbon-based polymers – burn easily.
Plastics that require Chloro-Flouro Carbons (CFC) during production may contribute to global warming when the CFC is allowed to escape. Thus, may lead to flooding in low lying areas.
Natural Polymers:
Natural Polymers are formed by condensation polymerization.
Protein:
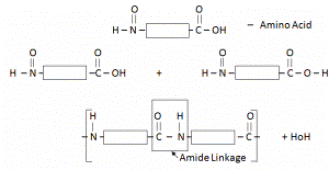
When two amino acids combine, protein is formed and H2O is a by-product.
Protein has a similar linkage to that of Nylon (amide linkage) –Thus can be called Polyamide.
They are joined together by condensation polymerization.
Each amino acid has an acidic functional group and an amino-functional group.
Proteins are possessing the same amide linkages as Nylon but with different monomer units.
Proteins can also by hydrated back into amino acids, by heating them with Sulphur Acid (H3PO4).
This adds water molecules into the polymer.
Read also: ELECTROLYSIS
Hydrolysis:
Is a reaction in the presence of alkali or acid that breaks down molecules by interaction with water?
The protein (amino-acids) and carbohydrate hydrolysis products can be chromatographically disassembled and described.
To become visible and compared to other pure monomer samples, a localizer (Ninhydrin) must scratch the chromatogram.
Carbohydrates:
Carbohydrates are polymers made up of small sugar molecules joined together e.g. starch.
Carbohydrates contain Carbon, Hydrogen, and Oxygen.
General Formula: CnC(H2O)n
Simplest Carbohydrate C6H12O6 (Glucose)
Glucose polymerase each other to form starch
Equation:
C6H12O6 (C5H10O5)n + nH2O
Hydrolysis of Carbohydrates to convert them to simple sugar:
Starch can be broken down into glucose (Simple Sugar molecules by heating with Sulphur Acid (Hydrolysis) – H2O molecule is added.
Chromatography can be used to identify small molecules of sugar
Fats:
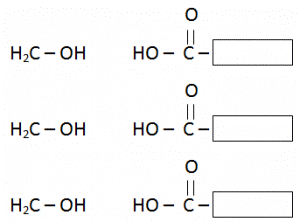
Fats are formed through a condensation reaction of fatty acids (Carboxylic Acids) and Glycerol (3 Carbon atom chains having OH attached with every Carbon atom).
H2O is also a by-product.
Fat is formed as a single large molecule so no need to write continuation brackets.
Glycerol contains three – OH functional groups per molecule and is hence known as a triol.
Each molecule of glycerol will combine with three molecules of fatty acids and form one molecule of fat.
Fats have the same esoteric linkages as Terylene (also known as polyester).
Fats can also be broken down into sodium salts of fatty acids and glycerol by boiling it with Acid or Alkalis (Hydrolysis).