What is Balancing Chemical Equations?
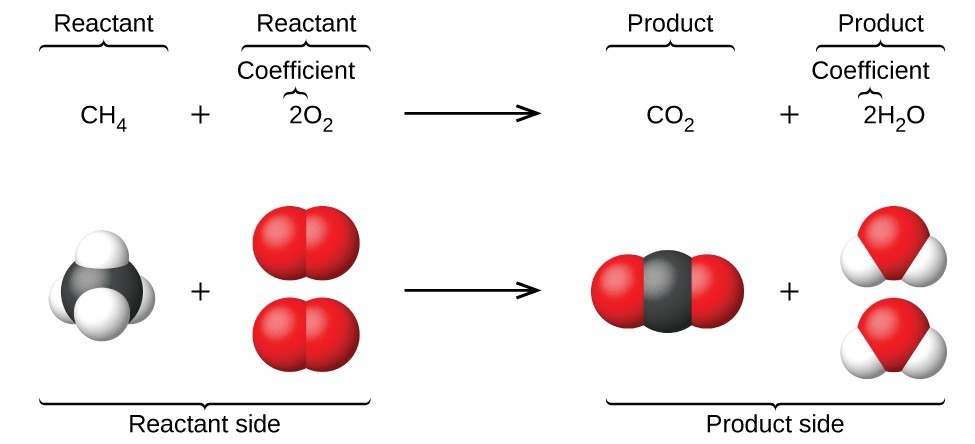
A balanced chemical is that the equation contains the equal number of atoms on each reactant and product side of each element involved in the reaction. This is a requirement that the equation must comply with the law of matter conservation.
Rules for Balancing Equations:
Rule 1: Look for an element that appears only once on the LHS and RHS
In other words, to start with, avoid elements in more than one compound. Why does this happen? Because each compound will require a coefficient that potentially unbalances other elements if an element appears in more than one compound. It is not a good idea to begin your balance by creating disequilibria.
When the element does not occur on both sides once and there is no chance, continue with the one that appears on the least of the compounds.
Rule 2: Try subscripts as balancing numbers
The coefficient on the one side of the equation will often be an element (or ion) subscript.
It’s the only trick you can learn. I predict that you’ll use this method to match 90% of calculations after going through more than 350 calculations to see what laws are used the most.
So, use subscriptions instead, you can’t change subscriptions.
Rule 3: Look for forced coefficients
When an element or ion exists on either side of the equation just once and is already balanced, the coefficient will be the same on both sides.
This rule also applies to polyatomic ions (e.g. SO42-, NO3–, CO32-, PO43- etc.)
Rule 4: Don’t start with compounds containing many elements
Usually, you should try to balance compounds that contain as few elements as possible first
Changing a coefficient in front of a compound containing three elements will potentially balance one element and unbalance two.
You are likely to be making life difficult for yourself. This rule is about keeping it simple. Of course, in some equations applying the earlier rules will need you to break this one.
Rule 5: Remember you can use fractions
Coefficients can be fractions and they are useful for correcting odd/even imbalances and adding single elements
Rule 6: Sum of charges LHS = sum of charges RHS
Correct negative charge imbalances using electrons.
Rule 7: Balance water later
Do not start with water unless the previous rules require you to.
During a chemical reaction, water is a very common product, and very common atoms are oxygen and hydrogen. Every amount you place on the water switches oxygen and hydrogen to make it possible to use a certain formula to match the elements of certain compounds.
The exception to this law is if you have a subscription to hydrogen or oxygen on one side of the equation that you choose to use as the element on the other.



