Chemical to Electrical Energy:
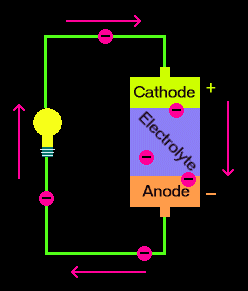
A cell or battery (a cell collection) is considered a tool that transforms chemical input into electric energy. It consists of an electrolyte with a pair with separate metals. The more reactive and ionized metal dissolves and produces electrons. The electrons then pass to the less reactive metal electrode and this electrode creates hydrogen bubbles.
This electrical output, or action, is electricity, such the electric energy is generated and the lamp the completes the circuit is illuminated and attached to the electrodes.
The irradiance of the bulb depends on the disparity between the two metals’ reactivities. The bulb is bright but not stays light for a long time because the metals are very different in the reactivity sequence. The bulb is less vivid but stays bright for a long time if the metals are similar in the reactivity zone.
By comparison to a solvent, since electrolytes in the cells become a paste, they become referred to as dry cells. Those are the most common forms of cells in the building. We are a convenient compact source of energy and are very inexpensive to buy.
Read also: ACIDS & BASES
Electrolysis
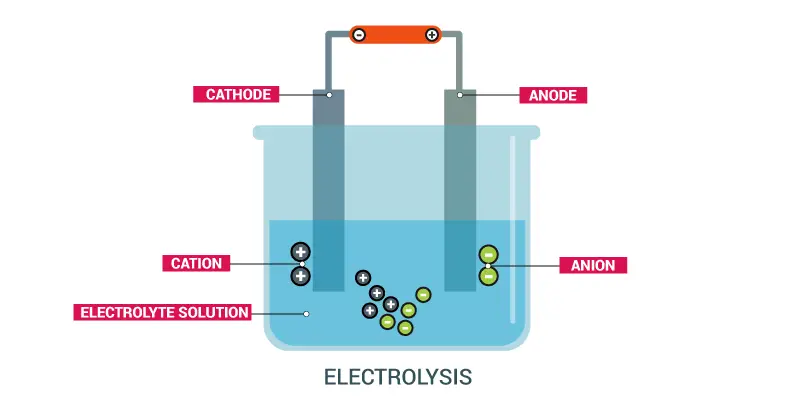
Electrolysis is the method of breaking down or decomposing a material using electricity. This is housed in an electrolyte cell that acts as an electric circuit and consists of three major components: a battery, electrodes, and electrolyte.
Electrodes are the plates that bring the electrical energy into the air. The cathode is the electrode attached to the cell ‘s negative edge. The anode is the electrode attached to a cell’s positive end.
Electrolytes are materials whose electrical current is disintegrated and broken down when melted or dissolved into water. Here, the passage of ions between electrodes transfers electricity through the air.
The carbohydrates, alkalis, or salts dissolved in water or molten salts are examples of electrolytes.
These are mostly ionic materials. A non-electrolyte is a liquid that doesn’t allow energy to flow through, and its sources are water and other covalent compounds without ions.
Process:
When an electrolyte experiences electricity, it undergoes a chemical breakdown. It breaks up the electrolyte. The ions move to the electrode that is charged. Negative ions (anions) lose their electrons in the anode at the anode, which is very happy for electron since it is charged positively and lacks the electrons. Positive ions (cations), which have an excess of electrons, gain electrons from the cathode, with a minimum negative charge. The release of ions at the electrode allows the electrolyte to decompose chemically.
This also allows electrons to pass between the cathode and the anode and enables energy to be transferred by ions during electrolysis.
Discharge is the method of removing or acquiring electrodes. Once electrodes are discharged, atoms or molecules are formed.
Factors Affecting Electrolysis:
For electrolyze, only one cation or anion is preferentially released if more than one form of cation or anion is present in a solution. It is called ion-selective entry.
Position in the Reactivity Series (for cations):
Because of other solution cations, the cations of an element smaller in the reactivity sequence are released at the cathode. This is because electrons are easier to take cations with a less reactive dimension.
Relative Ease of Discharge (anions):
The hydroxide ions give up electrons most easily, followed by iodide, bromide, and chloride ions. Remember that sulfate and nitrate ions will not be discharged during electrolysis.
Concentration:
When a certain ion’s concentration is high, the choice release can be impaired. This is expected to be produced with a higher concentration.
Type of Electrode:
Let’s take the aqueous (II) sulfate solution electrolysis as an example. If we use sugars, these electrodes are inert and do not affect electrolysis. Nonetheless, these are active electrodes that impact the electrolysis if we use copper electrodes. The copper electrode will melt in the anode, while the pink copper powder will deposit the copper ions on the cathode.
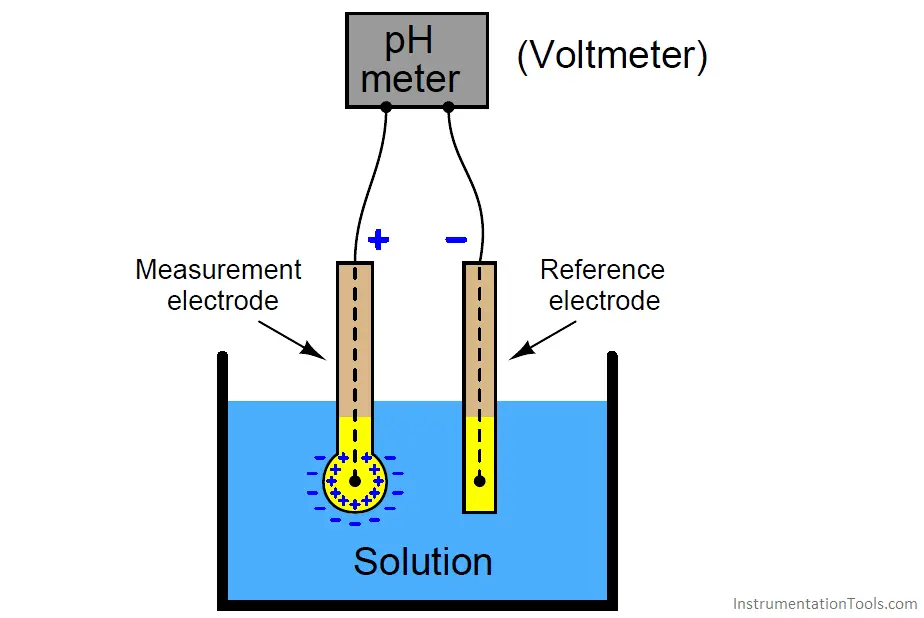
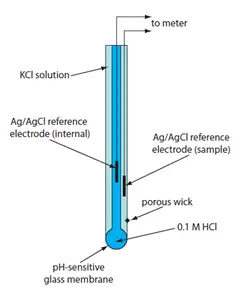
Type 1: The pH Electrode Principles:
Perhaps by using a glass electrode the most convenient and accurate way to determine pH. The pH electrode relies on the hydrated layers on the surface of the glass electrode.
This is a network of silicates containing metal ions coupled with oxygen and the metal ions interact with H+. Glass is a network of silicates. The glass electrode acts like a battery that has the voltage depending on the H+ activity of the solution.
Read also: Balancing Chemical Equations
The size of the potential (E) due to H+ is given by the equation:
E = 2.303 RT/F log ([H+]i/ [ H+]o
Where H+ is the inside and outside of the glass electrode with molar amounts with H+. [H+]; in reality, is normally 10-1, because the electrode contains 0.1 M HCL. Because pH=-log [H+] is, it follows that the potential produced for this solution is directly proportional to the pH outside the electrode. The lack of contact between components of the solution makes glass electrodes especially beneficial.
The internal reference electrode of the silver/silver chloride along the electrolyte of 0 is also present in the glass electrode.
The specific potential for the glass electrode can then be contrasted by combining an internal and external reference electrode with a steady potential provided by a conventional reference electrode, for example, the regular calomel electrode.
The calculated voltage is the product of the difference between the reference electrode and the glass electrodes. The potentials due to Ag / AgCl and the fluid junction with a reference electrode are also used, which provides the potential since the K+ and CI do not disperse at the same rate, creating a small potential at the boundary of the sample with the KCl of the reference electrode.
Therefore, the estimated potential for the glass electrode will also include the actual potential inside the unit.
Therefore, the equation becomes:
E = E* + 2.303 RT/F log ([H+]i/[H+]o),
where E* includes the standard electrode potential for glass electrode, and the constant junction potential present in the system.
Type 2: Ion Selective and Gas Sensitive Electrodes:
The glass pH electrode is a kind of ion-selective electrode that is sensitive to H+. Glass is the active material within the pH electrode, but modified aluminum silicate glasses can also be used to produce a variety of monovalent cation responsive electrodes. The ion-selective electrode responds to the activity of a particular ion. If some of the ions are not free and exist in a complex form or an insoluble precipitate, these electrodes will give a much lower reading, then with a method that detects all of the ions presents. A reference electrode is also needed with these ISE so that the varying potential of these ISE can be compared with the steady potential produced by the reference electrode.
Gas Sensing Electrodes:
These are commonly used to measure the amount of gas in a thin layer surrounding an ion-sensitive electrode, typically pH. The degradation into a thin film around the pH electrode and the resultant pH of the lying will both be determined by the carbon dioxide, sulfur dioxide, and ammonia.
Miniaturization and Applications of Ion Sensitive Electrodes:
ion selection electrode miniaturization was accomplished to react to different ions by modifying the field-effect Transit lenders. Such transistors (ISFETs) of selective ion field effects are probably of great clinical importance. The pH, Na+, K+, and Ca2 + are also used for multi-function ISFETs.