What are Redox Reactions?
A redox reaction is a chemical reaction in which electrons are exchanged between the two involved reactants. By examination of the changes in the oxidation states of the species interacting, this electron transfer can be established.
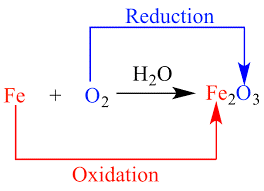
Below is a diagram describing the electron transfer in a redox reaction between two reactants.
Redox Reaction
The diagram above shows that the reactor an electron is pulled out of reactor Fe and that it is oxidized. Similarly, the reactant O was supplied with an electron and diminished as a result.
Oxidation is considered the electron loss and the resulting change in the oxidation rate of a certain reactant. The electron gain and the resulting reduction in the reactant’s oxidation state is considered a decline.
Organisms that accept electrons that are prone to redox reactions are considered oxidizing agents. The reduction agent can be called an electron-donating species that tends to hand out electrons.
These species are usually oxidizedEach redox response can be discarded into two parts, namely the half-reaction oxidation and the half-reaction reduction.
Any of them needs to be structured in a way to compensate for both electrons when writing these semi-reactions separately.
Types of Redox Reactions
The different types of redox reactions are:
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reactions
Read also: ATOMIC STRUCTURE
Decomposition Reaction
This kind of reaction involves the breakdown of a compound into different compounds. Examples of these types of reactions are:
2NaH → 2Na + H2
2H2O → 2H2 + O2
Na2CO3 → Na2O + CO2
All the above reactions result in the breakdown of smaller chemical compounds in the form of AB → A + B
But, there is a special case that confirms that all the decomposition reactions are not redox reactions.
For example, CaCO3 → CaO + CO2
Combination Reaction
These reactions are the opposite of decomposition reaction and hence involve the combination of two compounds to form a single compound in the form of A + B → AB
For example:
- H2 + Cl2 → 2HClC+O2→CO2
- 4Fe+ 3O2→2Fe2O3
Displacement Reaction
In this kind of reaction, an atom or an ion in a compound is replaced by an atom or an ion of another element. It can be represented in the form of X + YZ → XZ + Y. Further displacement reaction can be categorized into
Metal displacement Reaction
Non-metal displacement Reaction
Metal Displacement
In this type of reaction, a metal present in the compound is displaced by another metal. These types of reactions find their application in metallurgical processes where pure metals are obtained from their ores.
For example CuSO4+Zn→Cu+ZnSO4
Non-Metal Displacement
In this type of reaction, we can find a hydrogen displacement and sometimes rarely occurring reactions involving oxygen displacement.
Disproportionation Reactions
The reactions in which a single reactant is oxidized and reduced is known as Disproportionation reactions.
For example: P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Examples of Redox Reactions
A few examples of redox reactions along with their oxidation and reduction half-reactions are provided in this subsection.
Example 1: Reaction Between Hydrogen and Fluorine
In the reaction between hydrogen and fluorine, the hydrogen is oxidized whereas the fluorine is reduced. The reaction can be written as follows.
H2 + F2 → 2HF
The oxidation half-reaction is H2 → 2H+ + 2e–
The reduction half-reaction is F2 + 2e– → 2F–
The hydrogen and fluorine ions go on to combine to form hydrogen fluoride.
Read also: Redox Reaction
Example 2: Reaction Between Zinc and Copper
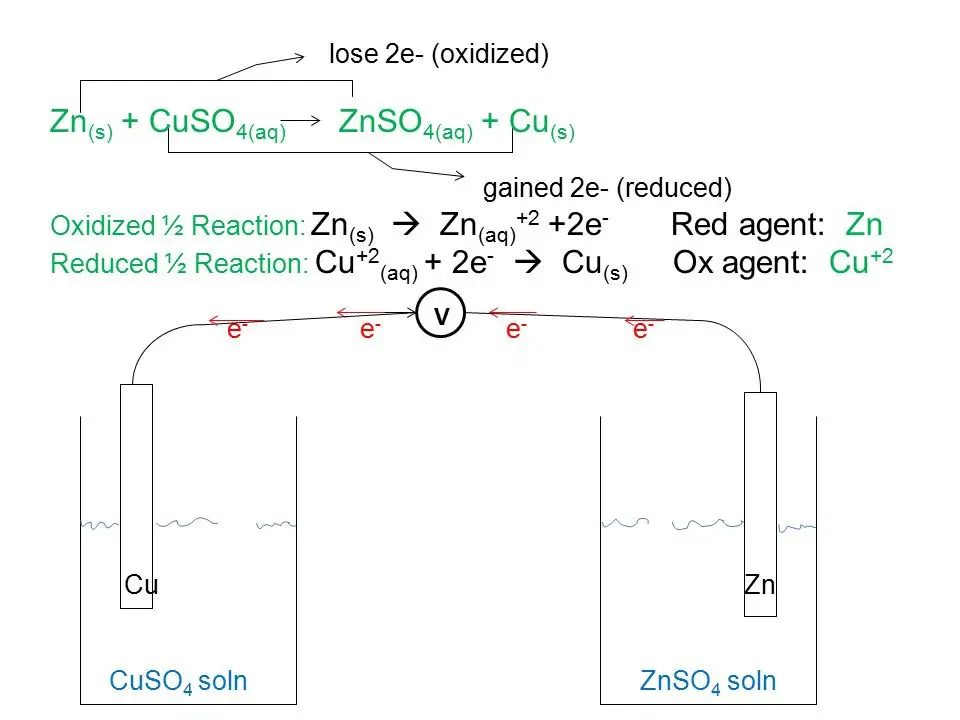
This is a type of metal displacement reaction in which copper metal is obtained when zinc displaces the Cu2+ion in the copper sulfate solution as shown in the reaction below.
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The oxidation h
alf-reaction can be written as Zn → Zn2+ + 2e–
The reduction half-reaction can be written as: Cu2+ + 2e– → Cu
Thus, copper is displaced from the copper sulfate solution by zinc in a redox reaction.
Example 3: Reaction between Iron and Hydrogen Peroxide
Fe2+ is oxidized to Fe3+ by hydrogen peroxide when an acid is present. This reaction is provided below.
2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
Oxidation half-reaction: Fe2+ → Fe3+ + e–
Reduction half-reaction: H2O2 + 2e– → 2 OH–
Thus, the hydroxide ion formed from the reduction of hydrogen peroxide combines with the proton donated by the acidic medium to form water.

