What are Atoms?
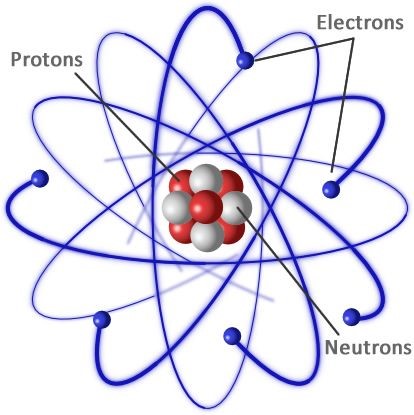
Atoms are composed of particles such as protons, neutrons, and electrons responsible for atomic mass and storage. The atom is the smallest material unit, maintaining all the element’s chemical properties.
Atoms shape molecules that interact with each other in the shape of solids, gasses, or fluids. Air, for instance, consists of atoms of hydrogen and oxygen that are mixed in water molecules.
Most molecular systems are structured to disintegrate molecules into their atoms so that they can be placed into a more usable molecule.
Read also: ATOMIC STRUCTURE
Atomic Models
Several physicists attempted to clarify the atomic structure using atomic simulations during the 17th and 19th centuries.
Each model had its strengths and demerits and played a critical role in the development of the modern atomic model.
Scientists John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr made the most important contributions to the field.
Dalton’s Atomic Theory
The English chemist John Dalton suggested that all matter is made up of atoms, which were indivisible and indestructible.
He also stated that all the atoms of an element were the same, but the atoms of different elements differ in size and mass.
Chemical reactions, according to Dalton’s atomic theory, involves a rearrangement of atoms to form products.
According to the postulates proposed by Dalton, the atomic structure comprised of atoms, the smallest particle responsible for the Chemical Reaction to occur.
The following are the postulates of his theory:
- Every matter is made up of atoms.
- Atoms are indivisible.
- Specific elements have only one type of atoms in them.
- Each atom has its constant mass that varies from element to element.
- Atoms undergo rearrangement during a chemical reaction.
- Atoms can neither be created nor be destroyed but can be transformed from one form to another.
Dalton’s atomic theory successfully explained the Law of Chemical Reaction, namely, the Law of conservation of mass, Law of constant properties, Law of multiple proportions, and Law of reciprocal proportions.
Atomic Number, Atomic Mass, and Relative Atomic Mass
A characteristic number of protons are present in the atoms of each element. The number of protons determines which atom we are looking at (e.g., all atoms of a six-proton structure are carbon atoms).
The number of neutrons, however, can vary for a specific element. Isotopes are called variants of the same atom and only vary in the number of neutrons.
Also, the photon count and the number of neutrons determine the mass count of an element: mass count = protons + neutrons. You will easily deduct the number of protons or the atomic number from the mass number anytime you choose to calculate how many neutrons an atom has.
The atomic mass of an atom is a property directly related to its mass number.
Atomic mass is the overall mass of a single atom and usually corresponds to atomic mass units or amu. Through nature, a six-neutron carbon atom, carbon-12, has a 12 amu atomic mass.
For reasons which go outside the scope of this paper, other atoms usually do not contain the round number of atomic mass. Nevertheless, as a general, the atomic mass of an atom is very similar to its height, but in decimal positions, it may have a difference.
Because the isotopes of the element have different atomic weights, scientists may often calculate the total atomic weight for an element, also referred to as the atomic weight.
The relative atomic mass is an estimate of the atomic masses of the different isotopes in a sample and the contribution of each isotope to the total sample is determined by the size of the fraction.
Of all-natural isotopes of each element, the total atomic mass in periodic table entries — including the one of hydrogen below — was determined, and are measured by the sum of such isotopes.
Extremely different isotope abundances can exist in alien objects, such as asteroids or meteors.
Read also: Redox Reaction
Atomic Structure of Isotopes
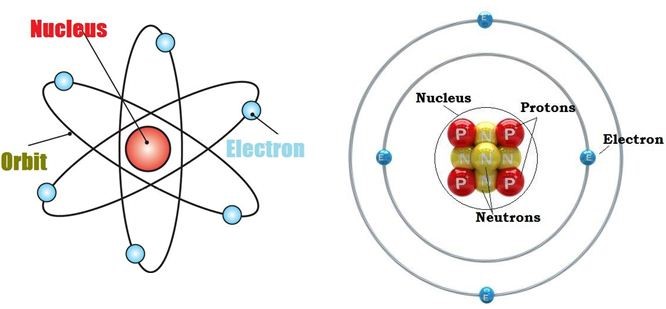
Nucleons are the components of the nucleus of an atom. A nucleon can either be a proton or a neutron. Each element has a unique number of protons in it, which is described by its unique Atomic Number.
However, several atomic structures of an element can exist, which differ in the total number of nucleons.
These variants of elements having a different nucleon number (also known as the mass number) are called isotopes of the element. Therefore, the isotopes of an element have the same number of protons but differ in the number of neutrons.
The atomic structure of an isotope is described with the help of the chemical symbol of the element, the atomic number of the element, and the mass number of the isotope.
For example, there exist three known naturally occurring isotopes of hydrogen, namely, protium, deuterium, and tritium. The atomic structures of these hydrogen isotopes are illustrated below.