What is Ammonia?
Ammonia, a distinct smelling colorless gas, is a chemical component and a key element in the production of many products used every day by people.
In the air, soil, and water and plants and animals, including people, it occurs naturally throughout the environment.
The human body produces ammonia when the body breaks down the protein-containing foods into amino acids and ammonia.
Read also: Redox Reaction
Reversible Reactions
Many chemical reactions can only go one way. I.e. they cannot and should not be changed. These reactions are known as irreversible.
For instance. Acid-alkali neutralization reaction
KOH (aq) + HCl(aq) + H2O(l) KCl(aq)
For instance. Reaction of combustion
2Mg(s) + O2(g) + 2MgO(s) Submitted
However, there can be several reversible chemical reactions. Instead of going towards completion, reactions can go to either direction and reach equilibrium. These reversible reactions are known.
In equilibrium, the reactions in front and back are not stopped, but at the same speed. Therefore, the number of reactants and products does not change in general. There are known to be a mixture of reactants and products.
A double flip sign — bar is used to display a reversible reaction.
The forward reaction is the left-to-right reaction.
Backward (or reverse) refers to the right-to-left reaction.
For example. Ammonium chloride, NH4Cl(s) thermal decomposition.
This is also a reversible reaction and the following equation is defined.
NH3(g) + HCl(g) + NH4Cl(s) + NH3(g)
When hot, solid ammonium chloride disintegrates into ammonia gas and hydrogen chloride gas.
Reaction forward: NH4Cl(s) + NH3(g)
Solid ammonium chloride is formed after refrigeration.
Backward reaction: NH3(g) + HCl(g) → NH4Cl(s)
There are many other examples of reversible reactions.
The reaction between nitrogen and hydrogen to form ammonia is another example of a reversible reaction.
Manufacturing of Ammonia by the Haber Process
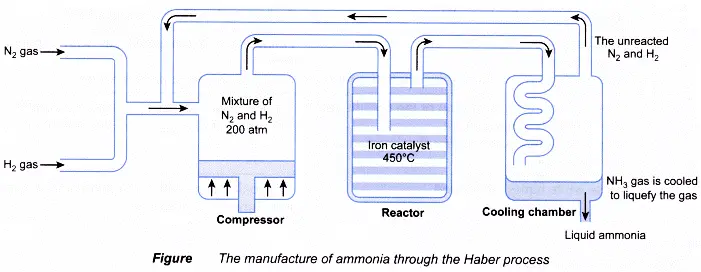
Ammonia is manufactured industrially by the Haber Process. The raw materials for the Haber Process are nitrogen gas and hydrogen gas.
Raw Materials:
N2(g) is obtained from the Fractional Distillation of liquefied air
H2(g) is obtained from the Cracking of crude oil (petroleum)
Equation:
N2(g) + 3H2(g) ⇌ 2NH3(g) ∆H = -ve
In reversible reactions, the forward and reverse reactions occur simultaneously.
Forward Reaction: N2(g) + 3H2(g) → 2NH3(g)
Backward Reaction: 2NH3(g) → N2(g) + 3H2(g)
Since the reaction is reversible, thus to have a good yield (amount) of ammonia gas, reaction conditions (temperature, pressure, and the use of catalysts) are carefully controlled.
Conditions:
The molecules of nitrogen and hydrogen are usually unreactive, and therefore, room temperature and pressure do not react between the two gasses.
A high pressure and a relatively high temperature are required for the two reactive gases to react together. Fine iron catalyst divided also is required to accelerate the chemical reaction.
As we have previously talked about, the reaction is reversible, i.e. certain of the formed ammonia molecules are decomposed into nitrogen and hydrogen. As such, chemical and chemical engineers control the reaction conditions (temperature and pressure) carefully so that the maximum production of the product (ammonia gas) is provided at minimal cost.
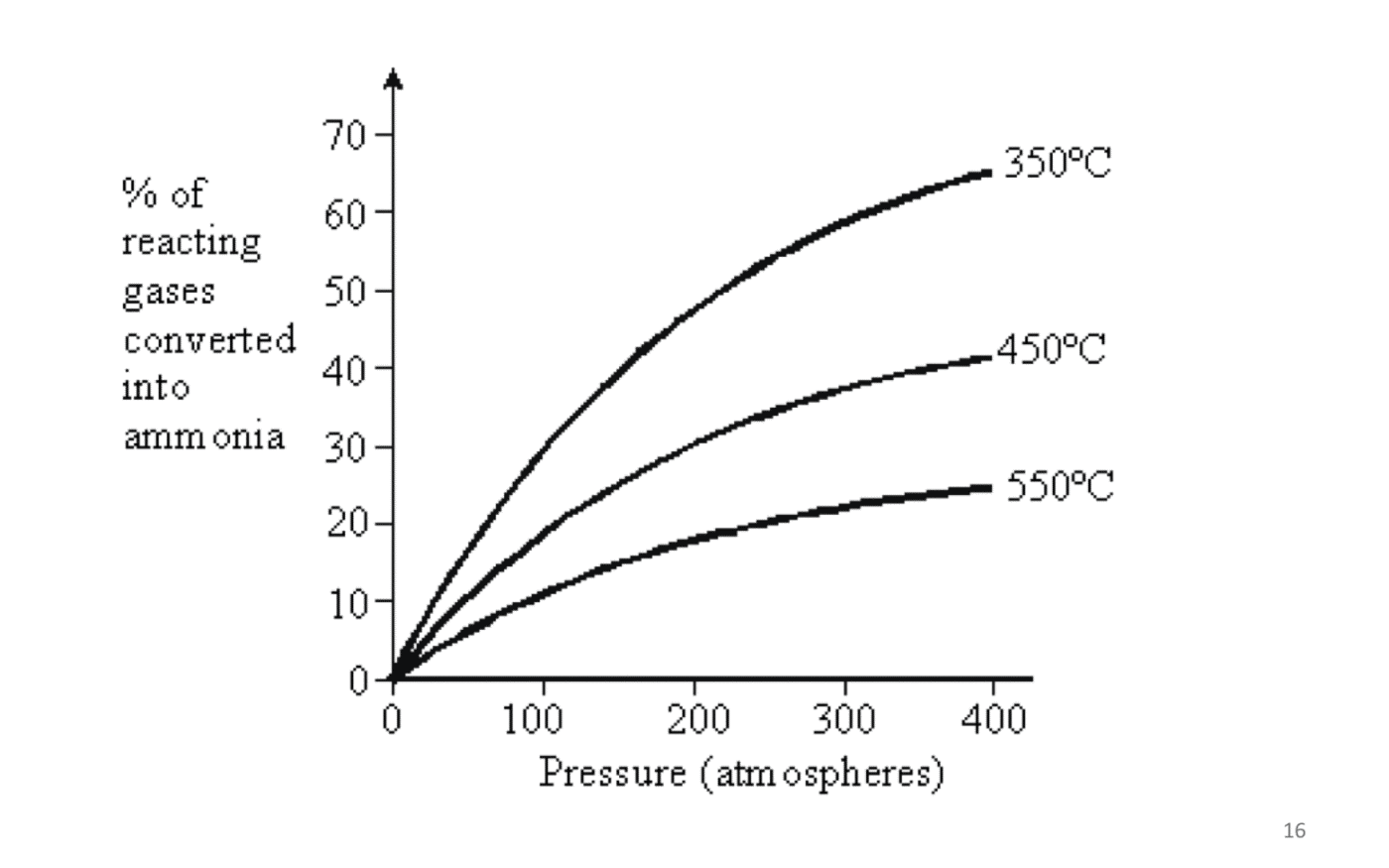
1) Effect of Temperature
The lower the temperature, the higher will be the yield of ammonia, since the decomposition of ammonia back to hydrogen and nitrogen will be reduced.
Lower temperature, however, will also results in a slower rate of reaction.
Therefore, there is an optimal temperature that is being used.
2) Effect of Pressure
The higher the pressure, the higher will be the yield of ammonia.
Higher pressure will also increase the rate of reaction.
However, the high-pressure conditions will be dangerous for people working in the manufacturing plant. More expensive equipment must be used to maintain high pressure.
Therefore, there is a limit to the amount of pressure that can be used.
Read also: ACIDS & BASES
3) Effect of Catalyst
Despite the high pressure and relatively high temperatures being used, the rate of reaction is still quite slow.
Therefore, a suitable catalyst is being used to speed up the rate of reaction.
Optimal Conditions in the Haber Process:
The best conditions used in the industry for obtaining the maximum yield of ammonia at minimum cost are:
The pressure of 200 atm
The temperature of 450 oC
Presence of a finely divided iron catalyst
Details in the Haber Process
- In 1:3 volume, nitrogen and hydrogen are mixed
- Gas compression to 200 atm and heating up to 450 oC
- The gas
- the mixture is passed through a finely divided iron catalyst
- Exothermal reaction
- Only 10-15% Nitrogen is converted to ammonia, that is to say, 10-15%.
- The conversion converter produces a mixture of nitrogen, hydrogen and ammonia
- Gas combustion is then cooled (condenser) in the cooling chamber
- Ammonia gas condensation into liquid ammonia while the gas remains hydrogen and nitrogen.
- Hydrogen and nitrogen pumped back into the converter for additional reaction (recycled) are not reacted.
Displacement of Ammonia from Ammonium Salts
Ammonia can also be prepared in the Chemistry laboratory.
When an ammonium salt is heated with a strong alkali such as sodium hydroxide, potassium hydroxide and calcium hydroxide, ammonia gas is being displaced from the salt.
Ammonium Salt + Strong Alkali → Salt + Water + Ammonia
e.g. NH4Cl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) + NH3(g)
Loss of Nitrogen from Fertilizers in Agriculture
Calcium hydroxide and calcium oxide (quicklime) are widely used in agriculture to neutralize excess acidity in soil.
However, both calcium hydroxide and calcium oxide will react with nitrogen fertilizers to form ammonia gas, which then escapes into the atmosphere.
e.g. 2NH4Cl(aq) + Ca(OH)2(aq) → CaCl2(aq) + 2H2O(l) + 2NH3(g)
This causes the loss of nitrogen from fertilizers already added to the soil by farmers.

